the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Analysis of a human-mediated microbioinvasion: the global spread of the benthic foraminifer Trochammina hadai Uchio, 1962
Mary McGann
Maria Holzmann
Vincent M. P. Bouchet
Sibelle Trevisan Disaró
Patrícia P. B. Eichler
David W. Haig
Stephen J. Himson
Hiroshi Kitazato
Jean-Charles Pavard
Irina Polovodova Asteman
André R. Rodrigues
Clément M. Tremblin
Masashi Tsuchiya
Mark Williams
Phoebe O'Brien
Josefin Asplund
Malou Axelsson
Thomas D. Lorenson
A non-indigenous species (NIS) of benthic foraminifera was first identified in a core collected in 1993 in San Francisco Bay, California, USA, and subsequently identified as Trochammina hadai Uchio, 1962. Archived samples and literature reviews were used to determine that the species, which is native to Asia, arrived in San Francisco Bay between the early 1960s and 1983. Through molecular analyses of specimens, archived samples and literature reviews from 1930–1983, and site surveys of harbors and estuaries along the western North American seaboard in 1994–2024, in total more than 2500 samples, we documented the presence of T. hadai at 73 locations in the USA and four in Canada. Trochammina hadai has also been recovered at nine sites in Sweden, two in France, three in Brazil, and two locations at one site in Australia. The rapid temporal and geographic spread of the NIS T. hadai in a non-native location is illustrated by a time series from 1930 to 2024 in San Francisco Bay. Between 1980 and 1986, the species' range expanded from low abundance (1.5 %) at a single site to cover nearly the entire South Bay with > 70 % abundance at some locations. By 1995 and continuing into 2010, the species expanded its range into the central and northern portions of San Francisco Bay, commonly with abundances of > 30 % and sometimes exceeding 70 %. This expansion may predate 1995, but a lack of samples makes it difficult to be more precise. Unfortunately, two Pb-210 and Cs-137-dated cores (BC01 and BC02) recovered from northern South Bay and Central Bay did not clarify this point, but additional cores may. Trochammina hadai is an infaunal opportunist that thrives in polluted locations. We surmise the species was introduced along the west coast of the USA in Puget Sound between 1902 and the 1920s, with cultivated oysters and oyster larvae and associated plant matter and residual sediment. This probably also happened in some areas of France, Sweden, and Brazil, where Japanese oysters were introduced in 1966, 1970, and 1975, respectively. After World War II, commercial shipping expanded dramatically and, with it, the release of ballast water and sediment in receiving ports, which introduced NIS worldwide. This primary vector of introduction occurred in large industrial harbors in several countries, sometimes followed by secondary introductions in small industrial centers and marinas by mud attached to the anchors and anchor chains of smaller boats.
- Article
(17665 KB) - Full-text XML
-
Supplement
(746 KB) - BibTeX
- EndNote
Biological invasions have greatly impacted ecosystems worldwide, particularly in urbanized ports of coastal bays and estuaries (Ruiz et al., 2000; Hewitt et al., 2004; Ruiz and Hewitt, 2009; Seebens et al., 2013). However, few studies have focused on the introduction of non-indigenous microorganisms (Drake et al., 2001; Ruiz et al., 2011; Stulpinaite et al., 2020), although they may impact ecosystem functioning (Fernandes et al., 2001) and include many human and animal pathogens (i.e., bacteria, particularly Vibrio cholerae O1 that causes human cholera, and viruses; McCarthy and Khambaty, 1994; Drake et al., 2001; Dobbs et al., 2023; Mimura et al., 2005; Oliveira, 2008; Emami et al., 2012).
Due to their small size, unicellular species are often overlooked in survey tracking of non-indigenous species (NIS) (Massé et al., 2023). For example, current ballast water regulations allow ships to discharge ballast waters with less than 10 viable organisms of the size range of 10–50 µm mL−1 (International Maritime Organization, 2004). Yet, unicellular species are the dominant biological component in ballast waters (Hülsmann and Galil, 2002; Granmo et al., 2018) and have juvenile and dormant stages (propagules) of just this size range (Alve and Goldstein, 2010). Hence, their propagules will be overlooked and disregarded as “viable organisms” by the ballast water regulations, despite the fact that they are able to arrive at new destinations where they may grow, reproduce, establish adult populations, and spread. Therefore, there is an urgent need to track these invisible invaders.
One of the earliest studies of non-indigenous microorganisms was of a single species of benthic foraminifera from the western coast of North America collected in 1946 that Harrington (1956) suspected was exotic. In collections from Tomales Bay, approximately 70 km north of San Francisco Bay (Fig. 1), he found several specimens of Ammobaculites exiguus Cushman and Brönnimann, in a mud sample collected in April 1946 from the edge of the tidal flat a short distance northwest of the town of Inverness. Harrington had never previously seen this species in his collections from Tomales Bay. Then in 1952 and 1955, he recovered Ammobaculites exiguus, Ammobaculites dilatatus Cushman and Brönnimann, and Miliammina (= Quinqueloculina) cf. M. fusca (Brady) near the 1946 location, as well as in the north side of the bay in 1955. Because the two species of Ammobaculites were so common in the mud of Tomales Bay in the early 1950s and were not mentioned in earlier surveys of the bay by Bush (1930) or Frank. B. Tolman (Recent littoral foraminifera of the central California coast, unpublished manuscript with checklist of foraminifera, 1933), Harrington concluded that they, as well as Miliammina cf. M. fusca, must have migrated or were introduced into the bay, possibly in about 1936 along with seed oysters (oyster larvae or “spat”) from Japan.
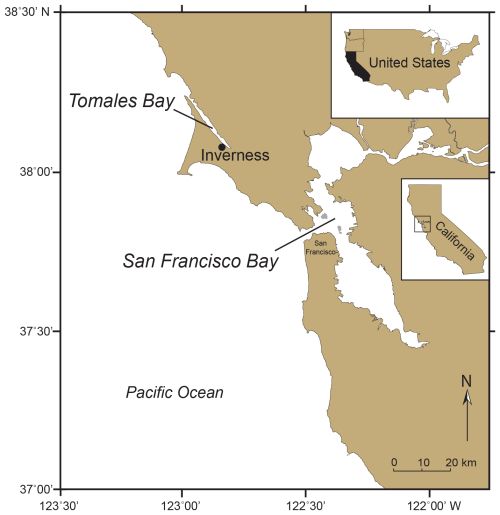
Figure 1Map of Northern California showing the locations of Tomales Bay and San Francisco Bay. The Tomales Bay town of Inverness is the site where Ammobaculites exiguus was first observed in 1946, making it possibly the earliest record of a suspected non-indigenous foraminifera in coastal western North America (Harrington, 1956).
The trend of not including microorganisms in NIS surveys has changed within the last 3 decades as non-indigenous benthic foraminifera have been reported in many regions worldwide. Most studies have focused on the “Lessepsian Invasion” where Red Sea organisms have entered the Mediterranean Sea through the Suez Canal (e.g., Bresler and Yanko, 1995; Hyams et al., 2002; Langer et al., 2012; Merkado et al., 2013; Weinmann et al., 2013; Stulpinaite et al., 2020), but other studies have reported NIS foraminifera in South America (Pupo and Disaró, 2006; Calvo-Marcilese and Langer, 2010; Eichler et al., 2018; Faria et al., 2021; Eichler, 2024), northern Europe (Bouchet et al., 2007; Schweizer et al., 2011; Polovodova Asteman and Schönfeld, 2016; Deldicq et al., 2019), the Adriatic Sea (Wiesner, 1911), Australia (Tremblin et al., 2021), Aotearoa / New Zealand (Hayward, 1997; Hayward et al., 1999), Canada (McGann and Holzmann, 2024), and possibly the northeastern Gulf of America (Moss et al., 2016). Richirt et al. (2021) found that in Great Britain an Asian phylotype of the genus Ammonia (i.e., Ammonia confertitesta Zheng; Hayward et al., 2021) was replacing the autochthonous phylotypes T1 and T2 (A. veneta Schultze, and A. aberdoveyensis Haynes, respectively; Hayward et al., 2004, 2021). Ammonia confertitesta has now been found as an invasive in Germany (Langer and Leppig, 2000; Ertan et al., 2004; Schweizer et al., 2011; Hayward et al., 2021), the Netherlands (Hayward et al., 2004, 2021; Richirt et al., 2019), Sweden (Bird et al., 2020; Polovodova Asteman et al., 2025), France (Bird et al., 2020, Pavard et al., 2023a, 2023b), and Canada (McGann and Holzmann, 2024).
Trochammina hadai may be the most comprehensively studied non-indigenous foraminifer. This benthic estuarine species is native to Asia (Fig. 2), originally described as “off River Shinano, Niigata-ken, Japan” by Uchio (1962) (Plate 1). Trochammina hadai has also been reported as indigenous from other sites in Japan (Morishima and Chiji, 1951; Matoba, 1970; Ikeya, 1977; Matsushita and Kitazato, 1990; Toyoda and Kitazato, 1995, and references therein) (Plate 2, Fig. 1a–b; Plate 3, Figs. 1a–2b); Jeju Island of South Korea (Choi and An, 2012; Kim et al., 2016); and China in the Bohai Sea portion of the Yellow Sea (Zheng and Fu, 1990; Lei and Li, 2016). We suspect that reports of the species' presence in India, noted as T. hadai in Rao et al. (2000), T. globigeriniformis (Parker and Jones) in Nigam and Thiede (1983), T. globigeriniformis var. pygmaea Höglund in Rao (1974), and T. globigeriniformis cf. globulosa Cushman in Seibold (1974), do not reflect native occurrences but a possible NIS invasion due to the widespread distance and lack of direct current exchange between the eastern coast of Asia and India.
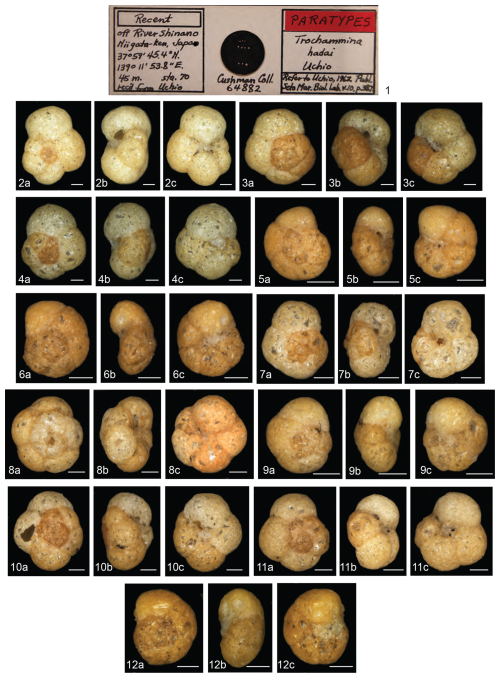
Plate 1Light microscope images of the 11 paratypes of Trochammina hadai originally described as “off River Shinano, Niigata-ken, Japan” by Uchio (1962). (1) Image of the T. hadai paratypes slide housed in the Cushman Foraminiferal Collection (64882) at the National Museum of Natural History, Washington, D.C., USA. (2–12) Spiral side (2a–12a), edge view (2b–12b), umbilical side (2c–12c). Scale bars: 100 µm. Note the morphological variability of the specimens illustrated. Photographs courtesy of Sibelle Trevisan Disaró.
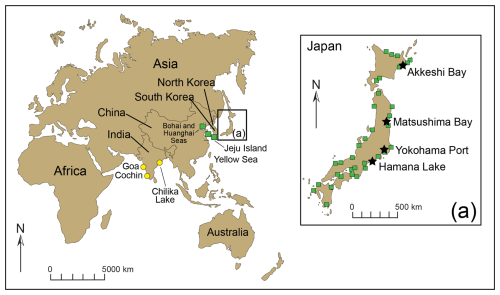
Figure 2Location of the benthic foraminiferal studies in Asia reporting the native occurrence of Trochammina hadai (green squares): in the Bohai Sea and Huanghai Sea portions of the Yellow Sea, China (Zheng and Fu, 1990); at Jeju Island, South Korea (Choi and An, 2012); and in Akkeshi Bay (Morishima and Chiji, 1951), Matsushima Bay (Matoba, 1970), Hamana Lake (Ikeya, 1977; Matsushita and Kitazato, 1990), and Yokohama Port (Toyoda and Kitazato, 1995, and references therein), Japan. (a) Japanese sites with foraminiferal assemblages characterized by > 50 % T. hadai are indicated by a black star. Species occurrences in Goa (Rao, 1974), Cochin (Seibold, 1974), and Chilika Lake (Rao et al., 2000), India, are not thought to be native distributions but NIS instead (yellow circles).
The first record of T. hadai as a NIS was in San Francisco Bay (McGann, 1995; McGann et al., 2000), a highly invaded estuary within a highly invaded bioregion (Cohen and Carlton, 1995, 1998; Ruiz et al., 2011). The species was found in the upper 20 cm of a core (DJ6-93SF-6) collected in 1993 in the western portion of southern San Francisco Bay (an embayment referred to locally as South Bay; Fig. 3) near San Francisco International Airport, at a water depth of 3.5 m (37.6305° N, 122.3650° W; U.S. Geological Survey Field Activity Number D-1-93-SF; McGann et al., 2024).
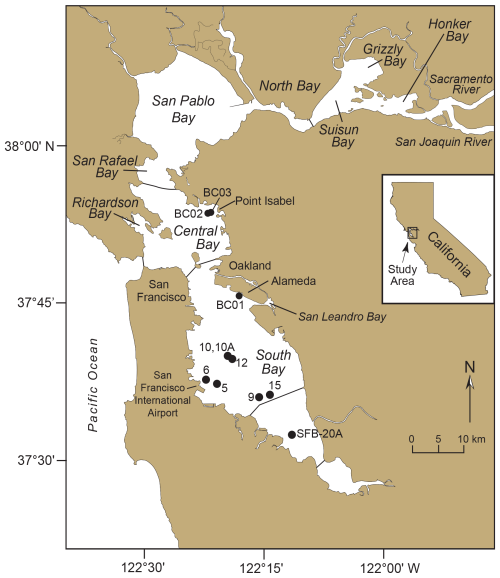
Figure 3Map of San Francisco Bay, California. San Francisco Bay consists of three embayments: North Bay (including San Rafael, San Pablo, Suisun, Grizzly, and Honker bays), Central Bay (including Richardson Bay), and South Bay. Cores 5, 6, 9, 10, 10A, 12, and 15 of the DJ6-93SF cruise obtained in 1993 are shown. Core 6 is the site where Trochammina hadai was first observed in San Francisco Bay. Core SFB-20A was analyzed at high resolution to assess its component stratigraphic signatures of the Anthropocene (i.e., a proposed geological epoch that describes the current time period where human activities have had a significant and lasting impact on Earth's geology and ecosystems) in the form of non-native species (Himson et al., 2023). Cores BC01, BC02, and BC03 were obtained in 2024 to determine when T. hadai spread into northern South Bay and Central Bay.
It has now been 3 decades since T. hadai was first recognized as a NIS in San Francisco Bay. The purpose of this study is to update the record of the species' distribution and abundance in western North America and the Hawaiian Islands, based on over 2500 samples collected between 1930 and 2024 (McGann et al., 2024). In addition, we document the recent molecular analyses of T. hadai specimens from the western coast of the USA, France, Sweden, and Australia, as well as earlier (1997, 1998) analyses from the species' native habitat in Japan. Finally, we discuss possible vectors, pathways, and spreading rates responsible for the dispersal of this global NIS.
2.1 Collection of benthic foraminifera
To update the occurrence of T. hadai in western North America and collect specimens for DNA analysis, 22 intertidal to shallow subtidal marine sites were sampled from Vancouver Island, British Columbia, Canada, to near the USA–Mexico border in May 2022 (U.S. Geological Survey field activity 2022-625-FA; Fig. 4a, d; Table 1). A single surface (0–3 cm) sediment sample, consisting of approximately 1800 cm3 of sediment, was obtained at each site using a large spoon in intertidal sites or a Petite Ponar Grab (Wildco) lowered off public docks from ca. 3 to 5 m depth at subtidal sites. The samples from each site were transported in containers placed in coolers filled with blue ice and frozen water bottles to the U.S. Geological Survey (USGS) Pacific Coastal and Marine Science Center (PCMSC) in Menlo Park, California, USA.
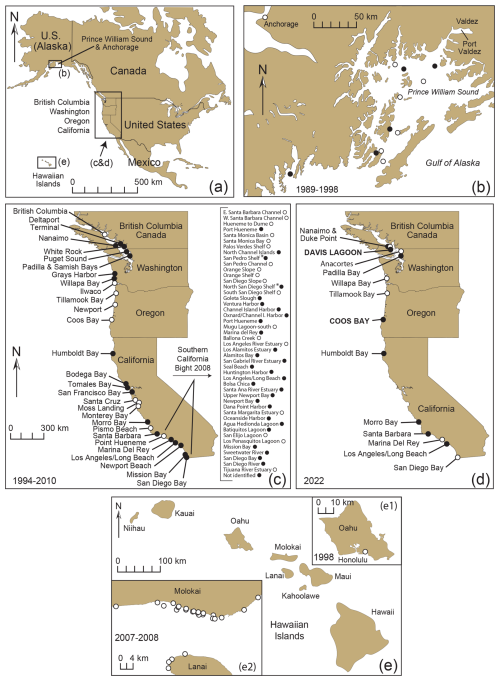
Figure 4Sites from western North America in the USA and Canada and the Hawaiian Islands used to investigate the presence of Trochammina hadai in surface or near-surface sediment samples (a). Locations collected in Prince William Sound, Alaska, in 1989–1998 (Quinterno and Carkin, 1991; McGann, unpublished data) and Anchorage in 1998 (McGann, unpublished data); (b) Nanaimo, B.C., Canada, to San Diego Bay, California, USA, in 1994–2000 (c) and 2022 (d); and the Hawaiian Islands (e) off Honolulu, Oahu, in 1998 (e1; USGS et al., 1998 and McGann, unpublished) and Molokai and Lanai in 2007 and 2008 (e2; McGann, unpublished data). Solid circles denote the presence of T. hadai in at least one sample per location; open circles denote the absence of the species in all samples at a location. Coordinates (latitude and longitude) were not available for all samples in Prince William Sound, Alaska. Data compiled in McGann et al. (2024).
Table 1Location and sampling date of sediment samples investigated in 2022 (USGS field activity number 2022-625-FA) for the presence of the non-indigenous foraminiferal species Trochammina hadai from southern Vancouver Island (Nanaimo), British Columbia, Canada, to San Diego Bay, California, USA. Asterisk denotes samples floated with the heavy liquid sodium polytungstate; bold font shows those locations in which T. hadai was found for the first time in 2022.
Approximately half of the container of sediment (0–3 cm) from each sample was separated out to isolate living specimens for molecular analysis. To remove clays, the sediment from these samples was washed with natural seawater using a hand-held pump sprayer through stacked 8 in. (20.32 cm) diameter 0.063 and 1.0 mm sieves. Sediment retained on both sieves was viewed under a Zeiss Stemi SV11 microscope to look for living specimens. Tests of Trochammina that appeared light yellow to orange in color (considered possibly alive) were transferred with a moistened picking brush to a petri dish filled with natural seawater. A small amount of washed sediment was added to the petri dish near the specimens and allowed to sit from a few hours to overnight, after which the specimens were viewed in the water with a microscope. Those with organic matter and/or sand grains attached to the aperture were assumed to be living and were removed with a picking brush, placed on a cardboard microscope slide, and dried at ambient temperature before being sent to the University of Geneva for molecular analysis.
The remaining half of the container of sediment from each sample (0–3 cm), from which the clay had not yet been washed, was wet-sieved with tap water through nested 8 in. (20.32 cm) diameter 0.063 and 1.0 mm sieves to remove silt and clay. Sediment remaining on the sieves was transferred to filter paper and air-dried. Samples retained on both sieves (very fine sand to coarse sand) that contained few foraminifers were floated in sodium polytungstate at a specific gravity of 2.3 g L−1 to concentrate the foraminifers (Parent et al., 2018). Because we sought only to determine the presence or absence of T. hadai, the samples were not split with a microsplitter or picked statistically. Instead, the dried sediment retained on the 0.063 and 1.0 mm screens was viewed under a microscope to determine the presence of T. hadai. The foraminiferal residues of this study are on file at the U.S. Geological Survey, Pacific Coastal and Marine Science Center, Santa Cruz, California, USA.
2.2 DNA extraction and PCR amplification and sequencing
Nine of the most robust-looking living T. hadai specimens submitted to the University of Geneva in 2022 from Padilla Bay, Humboldt Bay, Santa Barbara, and Los Angeles (isolates 21570-21572, 21575-21580) were photographed using a Leica M205 C microscope fitted with a Leica DFC 450 C camera at the University of Geneva prior to DNA extraction. DNA was extracted individually using guanidine lysis buffer (Pawlowski, 2000). Semi-nested PCR amplification was carried out for the 18S barcoding fragment of foraminifera (Pawlowski and Holzmann, 2014) using primers s14F3 (3'acgcamgtgtgaaacttg5')–sB (3'tgatccttctgcaggttcacctac5') for the first and primers s14F1 (3'aagggcaccacaagaacgc5')–sB for the second amplification. For the first and the second PCR, 35 and 25 cycles were performed, respectively, with annealing temperatures of 50 and 52 °C, respectively (Holzmann, 2024). The amplified PCR products were purified using the High Pure PCR Cleanup Micro Kit (Roche Diagnostics).
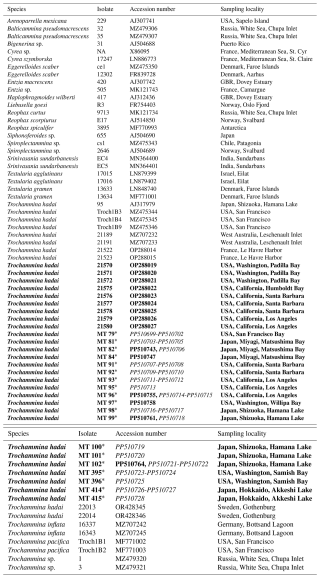
In total, 17 robust T. hadai specimens from the western coast of the USA (Samish Bay, San Francisco Bay, Santa Barbara, Los Angeles, and San Diego) and Japan (Hamana Lake, Akkeshi Lake, and Matsushima Bay) (isolates MT79, MT82, MT84, MT91-MT93, MT96-MT99, MT100-MT102, MT395-MT396, and MT 414-MT415) were submitted to the Japanese Agency for Marine Earth-Science and Technology (JAMSTEC) in 1997 and 1998 and selected for analysis using a stereomicroscope. DNA was extracted individually using DOC lysis buffer (Pawlowski, 2000). The 18S barcoding fragment was amplified using primer pair s14F1–sB. Amplification of the internal transcribed spacer (ITS) rDNA region was carried out for all T. hadai specimens extracted at JAMSTEC using the primer pair sBr (3'gtaggtgaacctgcagaaggatca5')–2TAIC (3'ctcactcgagctgatgtg5') (Pawlowski, 2000). ITS rDNA can be used to discriminate local populations and investigate intra-species relationships (Tsuchiya et al., 2003, 2014). For 18S rDNA and ITS rDNA amplification, 40 cycles were performed, with an annealing temperature of 55 °C (Tsuchiya et al., 2000, 2003, 2014). The amplified PCR products were purified using a MonoFas DNA Purification Kit (GL Sciences Inc., Tokyo, Japan) and then ligated into the pGEM-T Vector System (Promega, Madison, WI, USA) and cloned into XL-2 blue Ultracompetent Cells (Stratagene, La Jolla, CA, USA) or the pDrive Cloning Vector and cloned into EZ Competent Cells (Qiagen, Tokyo, Japan) according to the manufacturer's instructions. Plasmids were extracted using the QIAprep Miniprep kit (Qiagen). Sequencing reactions were performed for all obtained PCR products using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) and analyzed on a 3130XL Genetic Analyzer (Applied Biosystems). The resulting sequences from both the University of Geneva and JAMSTEC were deposited in the NCBI/GenBank database. Isolate and accession numbers are specified in Table 2.
Table 2Isolate, accession numbers, and sampling locations of analyzed species. Species in bold font have been investigated for the present study. Amplification products of isolates marked with an asterisk have been cloned prior to sequencing. Accession numbers in italics refer to ITS sequences. NA denotes not available.
2.3 Phylogenetic analysis
Of the sequences obtained for the 18S barcoding fragment of T. hadai, 15 were added to 27 sequences belonging to textulariids and seven belonging to T. hadai that are part of the publicly available 18S database of rotaliid foraminifera (NCBI/Nucleotide; https://www.ncbi.nlm.nih.gov/nucleotide/, last access: 20 May 2025). For the ITS rDNA region, 30 sequences were obtained. The sequences are based on cloned amplification products of 17 T. hadai isolates. One to four clones were sequenced per isolate.
Two different alignments were created for 18S and ITS sequences. All sequences were aligned using the default parameters of the Muscle automatic alignment option, as implemented in SeaView vs. 4.3.3. (Gouy et al., 2010). The 18S alignment contains 49 sequences with 1096 sites used for analysis. The ITS alignment contains 30 sequences, and 917 sites were used for analysis.
Phylogenetic trees were constructed using maximum likelihood phylogeny (PhyML 3.0) as implemented in ATGC: PhyML (Guindon et al., 2010) available online (http://www.atgc-montpellier.fr/phyml, last access: 14 May 2024). Automatic model selection by SMS (Lefort et al., 2017) based on Akaike information criterion (AIC) selected a GTR+G+I substitution model for the analysis of 18S barcoding sequences and a GTR+R model selected for the ITS analysis. Initial trees are based on BioNJ. Bootstrap values (BVs) are based on 100 replicates.
2.4 San Francisco Bay invasion chronology
We assembled the records of T. hadai presence or absence and abundance in San Francisco Bay from foraminiferal surveys conducted between 1930 and 2024 to create a time series in order to assess the timing of its arrival and spread in this non-indigenous location. Because the samples from 1986, 1987, and 1998 were recovered from long-term storage and analyzed years later, only dead counts were available for these time periods. Furthermore, only two samples from the 1995–1998 and 2014–2016 San Francisco Estuary Institute (SFEI) regional monitoring program (San Bruno Shoal and Richardson Bay sites in August 1995) and all of the sites from the 2010 bay-wide study were stained, although in the latter the living specimens were not abundant, which most likely reflected the January–February time of collection. To make the studies consistent, we only used the total (dead and living) counts in our time series.
To illustrate T. hadai's impact on San Francisco Bay's resident foraminiferal populations, we used Primer v. 7.0.23 statistical software (Primer-E, Ltd.; Clarke and Gorley, 2006) to conduct a rarefaction analysis (hypergeometric distribution for rarefaction; Hurlbert, 1971; Hayek and Buzas, 1997), excluding from the analysis subtidal marine taxa commonly found outside the bay because their occurrence likely reflects sediment transport into the bay (McGann et al., 2013) rather than the distribution of resident foraminifera. We used the results of the rarefaction analysis to determine a minimum sample size (i.e., > 200 specimens) to use with Surfer V6 software to produce contours illustrating T. hadai's relative abundance in the resident fauna.
To further refine the invasion chronology illustrated by the time series, three 6 in. (15.24 cm) diameter, 123.5–151.5 cm long bobcores (BC01, BC02, and BC03; U.S. Geological Survey field activity 2024-662-FA; McGann et al., 2024) were collected on 8 November 2024. Core BC01 was obtained south of Alameda in South Bay and Cores BC02 and BC03 in very close proximity to one another off Point Isabel in Central Bay (Fig. 3). The core sites were chosen because they were situated in depositional areas in San Francisco Bay (Fregoso et al., 2008), in regions of fine-grained sediment which are conducive to radiochemical analyses (Nittrouer et al., 1984; Wheatcroft and Sommerfield, 2005), located near the major shipping facility of the bay (i.e., Port of Oakland), and where they might further elucidate the timing of the spread of T. hadai into northern South Bay and Central Bay.
Upon their return to the USGS PCMSC Core Preparation and Analysis Laboratory in Santa Cruz, California, the cores were photographed, X-ray/computed tomography (CT)-scanned, and sampled for biology and radiochemistry. Age models for cores BC01 and BC02 were created based on radiochemical (Pb-210 and Cs-137) data, whereas no radiochemical analyses of core BC03 were conducted as this core was considered a close replicate of core BC02.
Lead-210 has a half-life of 22.3 years, thereby generally providing a steady-state chronology (Johannessen and Macdonald, 2012) for the past 100 to 120 years (Fuller et al., 1999; Alexander and Venherm, 2003; Alexander and Lee, 2009; Baskaran, 2011). Cesium-137 is a transient tracer (Johannessen and Macdonald, 2012) resulting from atmospheric nuclear testing and has a half-life of 30.0 years (Alexander and Venherm, 2003; Alexander and Lee, 2009). Chronologic markers using this isotope include (1) the onset of the Cs-137 record in 1954 due to initial bomb fallout, (2) the first fallout peak in 1959, and (3) another peak in 1963 after which input declined when atmospheric testing was banned (Johannessen and Macdonald, 2012; Drexler et al., 2018).
For Pb-210 and Cs-137 dating, 21 fine-grained (silt) sediment samples from core BC-02 were prepared following standard techniques described in Swarzenski et al. (2006) and Swarzenski (2014). Samples weighing 6–10 g were taken every 1 cm continuously down through 15.0 cm, every 2 cm from 16.0–21.0 cm, and then approximately every 5 cm to a depth of 37.0 cm (McGann and Lorenson, 2025). Similarly, 16 samples weighing 4–11 g were collected from core BC-01, but due to time constraints, the samples were taken every 2 cm down to 10.0 cm and then every 5 cm to a depth of 51.0 cm. Sediment samples for Pb-210 dating from both cores were weighed before and after drying at 105 °C for at least 24 h, disaggregated in a mortar and pestle, transferred to a plastic test tube, and then placed in a gamma radiation detector and counted. Total Pb-210 activities were directly determined by measuring the 46.52 KeV gamma peak using ORTEC High Purity Germanium (HPGe) radiation detectors. Supported levels of Pb-210 from Ra-226 were determined by measuring the gamma activity of Pb-214 (at 295 and 352 KeV) and of Bi-214 (609 KeV), the short-lived daughter products of Ra-226 (351.87 and 609.31 KeV). Cesium-137 activities in this study were determined by measurement of the 661.6 KeV gamma peak. Precision in these measurements was < 5 %.
3.1 Trochammina hadai distribution
Trochammina hadai was seen in samples from 10 of the 22 sites sampled in 2022 (Fig. 4d; Table 1). The species was recorded at two sites where it had not previously been reported: Davis Lagoon in southern Vancouver Island, British Columbia, Canada, about 30 km south of Nanaimo, where it had been reported in 1997 (Plate 4, Fig. 1), and Coos Bay, Oregon (Plate 4, Fig. 5) where it had not been seen in sampling in 1996, 1997, 1999, and 2000 (McGann et al., 2024). Trochammina hadai was not found in 2022 at three sites where it had previously been reported: Willapa Bay, Washington, and Marina del Rey and San Diego Bay in southern California.
3.2 Phylogeny
The 18S phylogenetic tree (Fig. 5) contains 49 sequences of agglutinated foraminifera of diverse ancestry based on their stratigraphic record and is unrooted. The obtained sequences cluster with T. hadai, supported by a high bootstrap value (BV) of 94 %. Trochammina pacifica Cushman (95 % BV), and Cyrea spp. (100 % BV) cluster at the base of T. hadai, but the group lacks a bootstrap support. Two other clades are present in the tree, supported by strong to moderate BV. One clade consists of Textularia gramen d'Orbigny, Bigenerina sp., Siphoniferoides sp., and Textularia agglutinans d'Orbigny (BV 96 %). The second clade contains Balticammina pseudomacrescens Brönnimann, Lutz and Whittaker, Entzia spp. and Arenoparrella mexicana Kornfeld, and is moderately supported (BV 77 %). A third clade without BV support contains Spiroplectammina sp. and Liebusella goesi Höglund, Srinivasania sundarbanensis Kaushik and Ghosh, Eggerelloides scaber (Williamson), and Trochammina inflata (Montagu) branch at the base of the latter three clades. Trochammina sp. branches at the base of all other textulariids. Species represented by more than one sequence are well supported by BV (86 %–100 %).
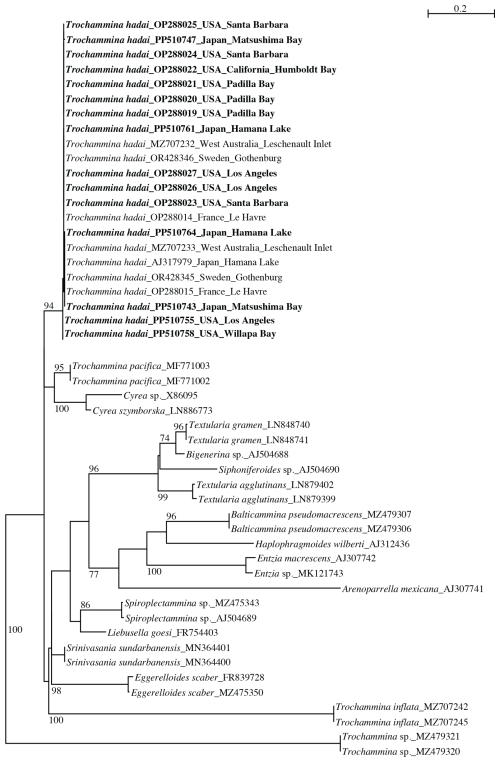
Figure 5PhyML phylogenetic tree based on the 3′ end fragment of the SSU rRNA gene, showing the evolutionary relationships of 49 foraminiferal sequences belonging to textulariids. Specimens marked in bold indicate those for which sequences were acquired for the present study. The tree is unrooted. Specimens are identified by their accession numbers. Numbers at nodes indicate bootstrap values (BVs). Only BV > 70 % are shown.
The ITS phylogenetic tree (Fig. 6) contains 30 sequences obtained from 17 T. hadai isolates and is unrooted. The tree is divided into two groups that lack BV support. One group contains 21 sequences obtained from 13 isolates; the other group includes 9 sequences obtained from 6 isolates. Two isolates (MT93, MT102) are represented in both groups. Sequenced clones MT93.1 and MT102.30 branch in the first group, while clones MT93.2 and MT102.31 are contained in the second group.
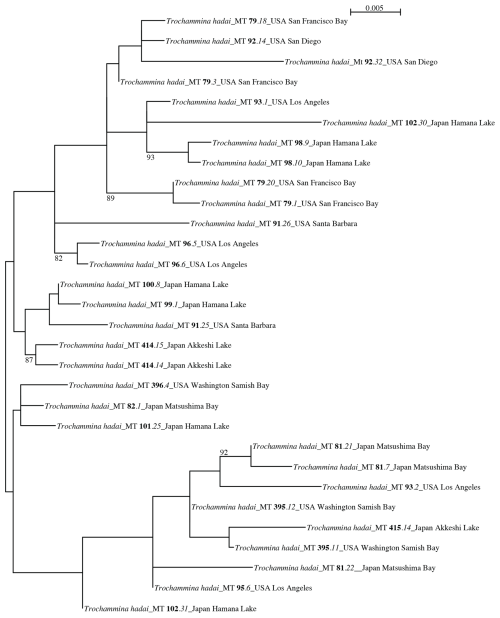
Figure 6PhyML phylogenetic tree based on the Internal Transcribed Spacer (ITS) region, showing the evolutionary relationships of 30 sequences belonging to Trochammina hadai. The tree is unrooted. Specimens are identified by their isolate numbers (marked in bold). Sampling locations are added to each isolate. Amplification products obtained for each isolate have been cloned prior to sequencing. Clone numbers are marked in italics. Numbers at nodes indicate bootstrap values (BVs). Only BV > 70 % are shown.
3.3 Chronology of cores
In core BC01 off Alameda, the Pb-210 record extended down to ∼ 51 cm; the depth at which unsupported (excess) Pb was absent was not reached (Fig. 7d). The concentration of Pb-210 was low, and the record proved to be somewhat noisy. Because of these factors, the Pb-210 accumulation rate was not used for the core chronology. The Cs-137 record was more binary, with Cs down to 25.5 cm and none recovered below that depth (Fig. 7e). The 1963 peak of Cs-137 occurred at 25.5 cm. A general sedimentation rate of ∼ 4.18 mm yr−1 for core BC01 was calculated based on sediment thickness between the Cs-137-derived 1963 time marker and the core top of 2024. Trochammina hadai was recovered in every 0.5 cm sample between the core top and 20 cm downcore, as well as from 25–26 and 30–31 cm (Fig. 7f).
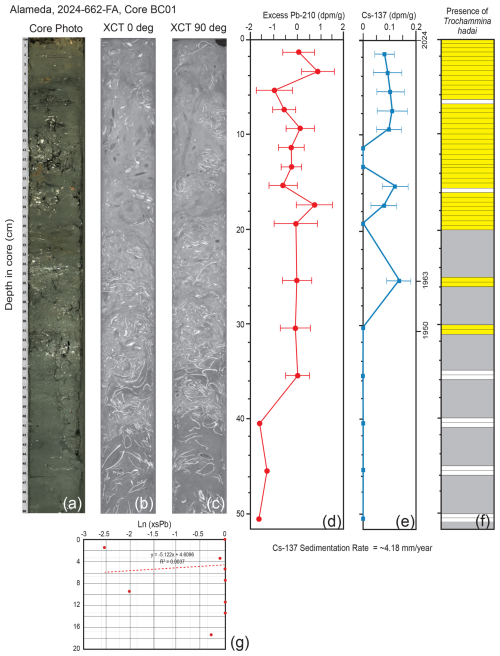
Figure 7Illustrations, radiochemistry, and presence/absence of T. hadai in the upper 50 cm of core BC01 collected off Alameda in 2024. (a) Core photograph. (b) X-ray/computed tomography (CT) image at 0°. (c) X-Ray/CT image at 90°. (d) Radiochemical profile of excess Pb-210. (e) Radiochemical profile of Cs-137. The 1950 calculated datum, the 1963 Cs-137 peak, and the core top of 2024 are labeled. (f) Presence/absence of T. hadai in 0.5 cm samples from 0–20.0 cm and then two adjacent 0.5 cm samples every 5 cm to a depth of 51 cm downcore. Yellow shading represents the presence of T. hadai, no shading represents its absence, and gray shading represents no sample taken. (g) Plot of sedimentation rates for bulk sediment calculated from the least-squares regression of the downcore excess Pb-210 activity data using the mass flux rate.
As with the Alameda core BC01 record, the overall concentrations of Pb-210 and Cs-137 were fairly low in Point Isabel core BC02, making the measurements less accurate. Once again, the trends were somewhat noisy, but at least a reasonable assessment of the sedimentation rate can be determined. The Pb-210 record extended down to ∼ 37 cm, and the depth at which unsupported (excess) Pb was absent was not reached (Fig. 8d). The Pb-210 trend line with an R2 of 0.63 results in a sedimentation rate of ∼ 1.78 mm yr−1. Cs-137 occurred down to 20.5 cm and then was absent below that depth (Fig. 8e). The earliest record of Cs-137 at 20.5 cm represents 1954. The maximum Cs-137 peak of 1963 is not clearly illustrated as there are four peaks between the depths of 14.5 and 5.5 cm. Using the core top to represent 2024, and either the 14.5 or 5.5 cm peaks to represent 1963, yields upper and lower limits for the sedimentation rates of 2.37 and 0.90 mm yr−1, respectively, with a mean value of 1.64 mm yr−1. The photograph of the upper 50 cm of the core shows no obvious stratification that can be tied to these results. However, if the point of no bioturbation is reached around 30 cm, then there is a possible tie to the Cs-137 profile where Cs is well mixed by bioturbation in the 0–30 cm interval. There also is mixing of the Pb-210 signal but not as much as that seen for Cs-137. Trochammina hadai was recovered in every 0.5 cm sample from the core top to 21 cm downcore and then again from 26.0–26.5, 31–31.5, 36.0–36.5, and 46.5–47.0 cm (Fig. 8f).
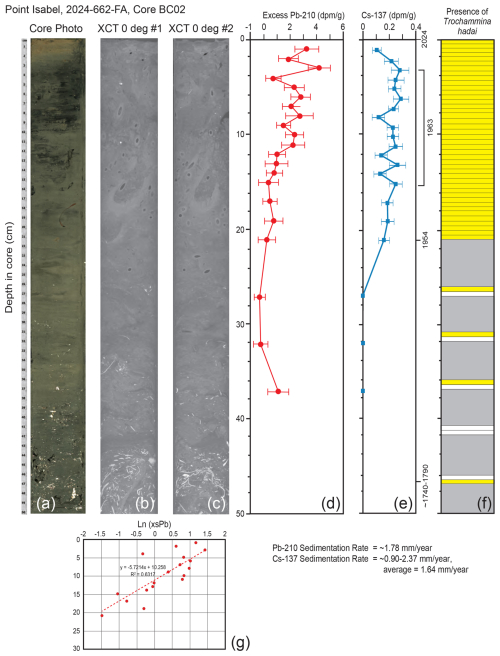
Figure 8Illustrations, radiochemistry, and presence/absence of T. hadai in the upper 50 cm of core BC02 collected off Point Isabel in 2024. (a) Core photograph. (b) X-ray/computed tomography (CT) image #1 at 0°. (c) X-Ray/CT image #2 at 0°. (d) Radiochemical profile of excess Pb-210. (e) Radiochemical profile of Cs-137. The ∼ 1740–1790 calculated datum, the 1954 Cs-137 first appearance, the 1963 Cs-137 peak range (5.5–14.5 cm), and the core top of 2024 are labeled. (f) Presence/absence of T. hadai in 0.5 cm samples from 0–21.0 cm and then two adjacent 0.5 cm samples every 5 cm to a depth of 47 cm downcore. Yellow shading represents the presence of T. hadai, no shading represents its absence, and gray shading represents no sample taken. (g) Plot of sedimentation rates for bulk sediment calculated from the least-squares regression of the downcore excess Pb-210 activity data using the mass flux rate.
4.1 Vectors of benthic foraminiferal invasions
Benthic foraminifera use their pseudopodia for locomotion, moving at a pace of only a few millimeters to up to 2.8 cm h−1 and migrating over only a few centimeters in a lifetime (Langer et al., 1989; Gross, 2000; Thibault de Chanvalon et al., 2015; Jauffrais et al., 2016). Clearly, this method provides a very limited dispersal mechanism and does not explain the long-distance translocations seen in some foraminiferal species such as T. hadai. Some species, however, form dormant resting stages and propagules (Alve and Goldstein 2002, 2003, 2010), the latter with a viable lifetime of months to 2 years (Alve and Goldstein, 2003, 2010). These have the potential to be transported long distances by currents, thereby lowering the threshold for colonization and expanding the species' geographic distribution.
Many other pathways for the introduction of NIS in marine settings have been demonstrated or postulated, most of which are anthropogenically mediated. Most commonly cited is the release of ballast water by commercial shipping vessels (Carlton, 1985; Williams et al., 1988; Carlton and Geller 1993; Smith et al., 1996; Chu et al 1997; Gollasch et al., 2000, 2002; Ruiz et al., 1997, 2000; Murphy et al., 2002; Dobbs et al., 2023; Radziejewska et al., 2006; Verna et al., 2016, 2021), especially in harbors and shallow bays. Ruiz et al. (2000) estimated that shipping and fishery practices together accounted for 89 % of the initial invasions in North America and 74 % of the repeated invasions. This is due to the immense volume of global maritime traffic and the large capacity of ballast water storage per vessel, which provides trim and stability during transit. David et al. (2015) estimated that 3.1×109 t (metric tons) of ballast water is transported around the world annually, and contained in this water is, among others, plant debris (diatoms, seeds, charophytes, spores, and pollen); multicellular aquatic organisms (amphipods, ostracods, mollusks, echinoids); protists (radiolarians, foraminifera, thecamoebians); and human-made objects such as microplastics, rope, metal chips, glass shards, welding slag, and micro-spherules (Carlton and Geller, 1993; Smith et al., 1996; Chu et al., 1997; Gollasch et al., 2000, 2002; McGann et al., 2019a). Although many countries require ballast water exchange (BWE) commonly ≥ 200 nmi from land and in water ≥ 2000 m deep (e.g., Canada, Minister of Justice, 2022) to lessen the threat of introducing NIS, large quantities of ballast water are still discharged into receiving ports: 116×106 t was discharged into Canadian ports during a 12-month period (data combined estimates from 2006, 2007, and 2008; Casas-Monroy et al., 2015), 125×106 t into US ports during a 3-year period (1 January 2005 to 31 December 2007; Miller et al., 2011), and 55×106 t into San Francisco Bay alone during a 9-year period (2006 to 2014; Verna et al., 2021).
Offshore BWE does not always eliminate the potential threat of introducing NIS either. During the uptake of ballast water in shallow ports, sediment may be re-suspended by the pumps and taken up with the water, entraining foraminifera, if present, in the process. This would be especially likely for foraminifera such as T. hadai, that live in the uppermost centimeters of the sediment column (Matsushita and Kitazato, 1990; Tremblin et al., 2021; Fig. 9). That which remains in suspension may be released with the water in receiving ports and may contain foraminiferal propagules (Alve and Goldstein, 2002, 2003, 2010) and/or tests (Carlton and Geller, 1993; Chu et al., 1997; Galil and Hülsmann, 1997; Gollasch et al., 1998; Macdonald, 1998; Lavoie et al., 1999; Smith et al., 1999; McGann et al., 2003, 2019a). Similarly, the sediment that settles in the tanks provides a suitable habitat for organisms to live and reproduce with the potential to be released later (Waters et al., 2001; Bailey et al., 2005; Duggan et al., 2005, 2006), making all previous ports of call by a ship a potential source of invasive organisms (Seebens et al., 2013; McGann et al., 2019a). Although many of these ships are referred to as No-Ballast-On-Board (NOBOB) when they arrive at a port, the configuration of the pumps is such that they cannot remove all the sediment, and the ballast tanks are only routinely cleaned approximately every 2 years, although more often when there is substantial build-up of sediment (U.S. EPA et al., 2017). The residual sediment may contain fish, crabs, and other invertebrates, as well as abundant and diverse foraminifera, many of which have a substantial living component (McGann et al., 2003, 2019a). For example, ballast sediment from 9 of 11 vessels arriving in Prince William Sound from domestic (San Francisco Bay and Puget Sound) and foreign (Japan, South Korea, and one unspecified) ports contained T. hadai (McGann et al., 2019a); 2 of 16 vessels arriving in the Great Lakes of the USA also contained the species, one that had traveled from Ghent, Belgium, and Teurneuzen, the Netherlands, and the other from Ijmuiden, the Netherlands (Thomas Johengen, University of Michigan and National Oceanic and Atmospheric Administration, personal communication, 2001 and 2002). Furthermore, McGann et al. (2019a) estimated that this vector could have been responsible for the introduction of as many as 440 billion to ∼ 9 trillion living foraminifera per year into Prince William Sound.
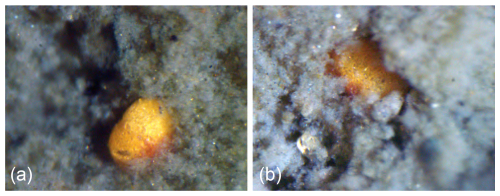
Figure 9Live Trochammina hadai partly exposed at the top of a flocculent mud layer from Leschenault Inlet, Australia. Note that the aperture and umbilical side faces downward in the sediment, optimally positioning the pseudopodia in the vicinity of the sediment to enhance feeding (Denton et al., 2025). Photographs courtesy of Clément Tremblin and David Haig.
Other vectors are far less significant overall in the introduction of NIS but may be the major contributor in localized regions. Commercial mariculture, such as oyster and mussel culturing, unintentionally transports living organisms attached to their shells, among the plant matter used as packing material, or loose in the associated residual sediment, to the receiving farming site where they may flourish and spread (Carlton, 1979). For example, Atlantic oysters (Crassostrea virginica (Gmelin)) were first transplanted into San Francisco Bay from New York and Baltimore in 1869 or 1870 (Kornicker, 1975) after the completion of the transcontinental railroad in 1869 (Sculati, 2004; Williams, 2005). By 1875, large quantities of seed oysters were imported, and the practice was continued until 1910 (Kornicker, 1975). In the late 1890s, over 250 train loads (about 9000 barrels) of oysters arrived each year to be transplanted into San Francisco Bay (Kornicker, 1975: Sculati, 2004). Many macroinvertebrates (Cohen and Carlton, 1995) and an ostracod (Eusarsiella zostericola (Cushman); Kornicker, 1975) were introduced using this method, possibly also the foraminifera Haynesina germanica (Ehrenberg), which occurred consistently in core DJ6-93SF-6 (Fig. 3) from an estimated time of about 1868 to the core top in 1993 (McGann, 2008). This species is a native along European coastlines and in the Mediterranean Sea (Murray, 1991; Calvo-Marcilese and Langer, 2010). Similarly, as mentioned earlier, the presence of Ammobaculites exiguus, Ammobaculites dilatatus, and Miliammina cf. M. fusca in Tomales Bay may have been due to seed oysters introduced there possibly in 1936 from Japan.
Seaweed or other marine plant matter packing used to transport live bait worms from the east coast of the USA to the west coast overnight by aircraft also has been shown to include viable benthic foraminifera (McGann, unpublished data). The specimens are dumped along with the unused plant matter into western ports and harbors by fishers within hours of their eastern departure (Lau, 1995; Cohen et al., 2001), thereby providing a nearly instantaneous mechanism by which cross-continental introductions may occur.
Fouling on hulls of ships and boats, floating debris (seaweed, mats of shallow-water vegetation, coconuts, logs, volcanic pumice), and human-made objects such as plastic debris and tsunami-generated debris rafting (e.g., large docks, small buoys and boats, totes, and fishing gear), including those that were carried by currents from Japan to the west coast of North America and the Hawaiian Islands as a result of the Tohoku earthquake and tsunami of 2011 (as well as previous occurrences in 1611, 1896, 1933, and 1960 from the Valdivia earthquake), are other vectors by which small numbers of foraminifera may be transported to distant locations (Richards, 1958; Jokiel, 1984; Carlton and Hodder, 1995; Winston et al., 1997; Gollasch et al., 1998, Rowlands, 2007; Gollasch, 2010; Jorissen, 2014; Carlton et al., 2017; Finger, 2018; Subías-Baratau et al., 2022). Most fouling foraminifera are common epifaunal taxa (e.g., Tretomphalus bulloides (d'Orbigny), Rosalina globularis (d'Orbigny), Planogypsina acervalis (Brady), and Cymbaloporetta bradyi (Cushman), among others; Rowlands, 2007; Jorissen, 2014; Finger, 2018; Subías-Baratau et al., 2022), with species displaying preferences for the substrate to which they will attach including macroalgal coverings, calcareous surfaces, and even boring into cement (Rowlands, 2007).
One of the best ways to prevent NIS introductions is to manage their vectors, and a good example of this is in the difference between hull fouling management measures used on large commercial vessels versus small pleasure boats (Mineur et al., 2008; Murray et al., 2014; Murray et al., 2011; International Maritime Organization, 2024). Although large transoceanic vessels are required to apply mandatory antifouling compounds such as TBT (i.e., tributylin; International Maritime Organization, 2004), small recreational and fishing boat traffic is largely exempt from these regulations (Murray et al., 2011). This fact, combined with the latter's ability to travel long distances at a relatively low speed, makes recreational and fishing boats perfect vectors for fouling NIS (Minchin et al., 2006). As their travel routes are harder to identify compared to transoceanic vessels, it also creates highly unpredictable chances of secondarily spreading from the first introduction hotspot (e.g., an international trade harbor) to other close locations such as leisure boat harbors. Additionally, if a NIS invasion is successful, their introduction site could serve as a nearby reservoir of NIS, facilitating more regional spreading (Bertelsmeier et al., 2018). This is especially true if propagules are released that are capable of remaining viable in unsuitable settings that are not normally occupied by the adults (Weinmann and Goldstein, 2017). Trochammina hadai has not been identified as an epibiont species; therefore hull fouling or attachment to other floating objects may not be an applicable vector for the species.
Sediment clinging to anchors and anchor chains may transport foraminifera to distant ports when they are dropped, retrieved, or cleaned there (Carlton et al., 1995; McGann, unpublished data). Although not nearly as large a scale as the release of ballast sediment, this vector may be one means by which secondary introductions of NIS occur in small leisure boat harbors that are not directly subject to transoceanic vessel traffic (see Eichler et al., 2018; Polovodova Asteman et al., 2025).
Of far less importance may be the occurrences of foraminifera being transported to new locales as research escapes (Ruiz et al., 2000); as ornamental escapes (Cohen and Carlton, 1995; Ruiz et al., 2000); and on birds' feet, bills, and feathers, especially when encapsulated in the mud coating on these (Headlee, 1961; Resig, 1974; Patterson, 1987; Hayward et al., 1999; Riedel et al., 2011; Tremblin and Walker, 2025). Foraminifera have also been reported within the guts of shorefish (Todd, 1961), rabbitfish from the Red Sea that were NIS in the Mediterranean Sea (ichthyochory; Guy-Haim et al., 2017), holothurians (Goldbeck et al., 2005), gastropods (Hickman and Lipps, 1983), and birds (Jere Lipps, University of California, Berkeley, personal communication, 2019); all of these examples are thought to reflect passive ingestion of living foraminifera while eating other food sources with the eventual defecation of viable specimens, except for the neogastropod Olivella, which selectively ingests foraminifera (Hickman and Lipps, 1983).
In addition to all the vectors listed above, anthropogenic construction projects and climate change have opened pathways for NIS introductions. These have reduced physical barriers (e.g., the Suez and Panama Canals; Katsanevakis et al., 2020) and thermal barriers such as the new shipping passages that have emerged in the Arctic due to melting ice caps (e.g., the route between Svalbard and Japan along the northern coast of Russia; Ware et al., 2016; The International Union for Conservation of Nature, 2017; Thyrring et al., 2017; Nong et al., 2019), thereby greatly reducing transit times and breaching of previously limited habitats, allowing for range expanses of NIS (de Rivera et al., 2006; Thomas et al., 2008) and the outpacing of native species.
4.2 Proliferation
To illustrate the temporal and geographic spread of T. hadai in a non-native location of the US Pacific coast, a time series was constructed using 1110 archived sediment samples, as well as literature documenting abundances and distributions of benthic foraminifera in surface sediments in San Francisco Bay from 1930 to 2024 (McGann, 2024; McGann et al., 2024). From 1930 to 1981, 430 samples from 11 studies documented the absence of the exotic species throughout the bay (Fig. 10a–d; McDonald and Diediker, 1930; Conomos, 1963; Means, 1965; Slater, 1965; Gram, 1966; Quinterno, 1968; Locke, 1971; Connor, 1975; Wagner, 1978; James Ingle Jr., Stanford University, personal communication, 1995; Doris Sloan, University of California, Berkeley, personal communication, 1997). In April 1983, T. hadai first appeared, albeit rarely (12 specimens, comprising 1.5 % of the assemblage) at one of four sites sampled in South Bay (Fig. 10e; McGann, 2014). The timing of this first appearance is corroborated by the first appearance of the non-indigenous ostracods Spinileberis quadriaculeata (Brady) and Bicornucythere bisanensis (Okubo) in core SFB-20A obtained in South Bay in 2019 (Fig. 3; USGS field activity 2019-616-FA), as both are thought to have been introduced into the bay in 1975–1976 and appear stratigraphically below the first appearance of T. hadai (Himson et al.). Just 3 years later (1986) the species had increased dramatically within South Bay both in geographic extent and abundance (Fig. 11a; mean 42.7 %, maximum 88.7 %; McGann, 2018) and remained a significant species in the area in 1987 (Fig. 11b; mean 40.6 %, maximum 83.0 %).
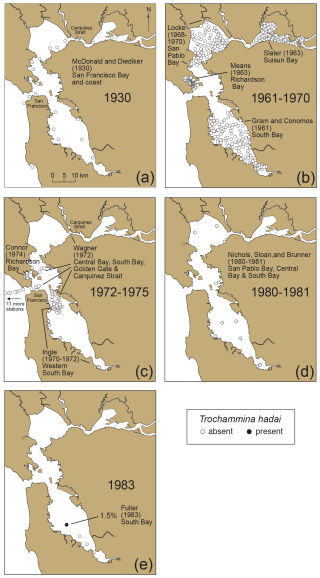
Figure 10Presence and absence of Trochammina hadai in San Francisco Bay from 1930 to 1983. Data based on dead specimens only. (a) 1930, (b) 1961–1970, (c) 1972–1974, (d) 1980–1981, and (e) 1983. Sample collector and the year of collection (not publication) listed for each area (see McGann et al., 2024). The solid black circle (in 1983) denotes the site of the first recovery of T. hadai in San Francisco Bay, and open circles denote the absence of the species. Note that 11 of Wagner's samples (c) are located to the west outside the bay, off the map.
After T. hadai was first recognized as a NIS in the bay in the 1993 South Bay core DJ6-93SF-6 (Fig. 3), we checked other South Bay cores from cruise DJ6-93SF (i.e., cores 5, 6, 10, 10A, 12, and 15; Figs. 3, 11c; McGann et al., 2024) and found T. hadai in all the core tops and as deep as 30 cm downcore (core 10). The abundances ranged from 8 % to 56 %, the latter occurring in core 10 as well. We then partnered with SFEI, joining their bay-wide monitoring program. Bi-annual samples, 232 in all, were obtained throughout the bay for the next 4 years (1995–1998; McGann et al., 2024). With the wider geographic coverage, the proliferation of T. hadai into the central and northern portions of San Francisco Bay (hereafter referred to by the local names “Central Bay” and “North Bay,” respectively) was evident (Fig. 11d–i). The highest abundances occurred in eastern Central Bay and the northern portion of South Bay where the species comprised > 70 % of the fauna at several locations, with nearly monospecific assemblages at a few sites (86.7 %–97.4 %). Only the extreme southern end of South Bay, as well as North Bay from Honker Bay to the eastern portion of San Pablo Bay, was exempt from the invasion. We attribute the absence of T. hadai in these locations to the influx of freshwater entering from local streams in South Bay and the major Sacramento and San Joaquin Rivers (Fig. 3) in North Bay. The latter two drain a large portion of central California, often pushing the boundary between freshwater and seawater (the 2 psu line) from Honker, Grizzly, and Suisun bays during the summer into San Pablo Bay during the winter (McGann et al., 2013; Fig. 11d, e, g, i), making the environment unhabitable for most foraminifera, including T. hadai.
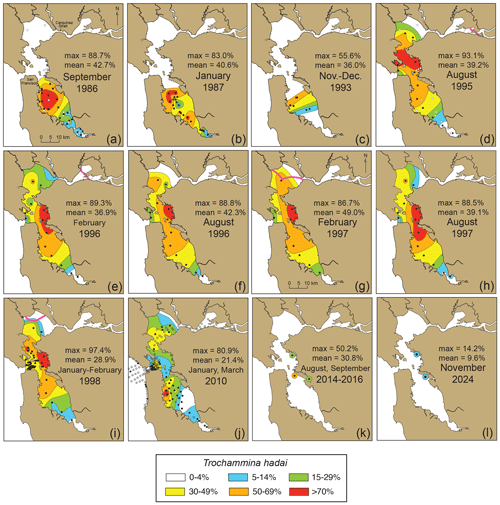
Figure 11Time series of the distribution of total (living and dead) Trochammina hadai in San Francisco Bay. (a) September 1986; (b) January 1987; (c) November–December 1993; (d) August 1995; (e) February 1996; (f) August 1996; (g) February 1997; (h) August 1997; (i) January–February 1998; (j) January and March 2010; (k) August 2014, September 2015, and August 2016; and (l) November 2024. Note the absence of samples taken in western Central Bay in 2010. The distributions are based on single samples (no replicates) and may be affected by increased productivity of other species, taphonomic loss, and seasonal blooms, although the latter is less likely due to sampling in successive summers and winters (September 1986 to January 1987 and August 1995 to January–February 1998). Solid black circles denote the presence of T. hadai. In maps (a) and (d), the open circles denote, for comparison, the location of sites in South Bay in 1980–1981 and 1983 (see Fig. 10d and 10e) where no T. hadai was recovered. The boundary between freshwater and seawater (the 2 psu line), if present, is shown in the North Bay as a heavy pink line. In summer, the 2 psu line was located in Honker Bay and the San Joaquin River (August 1995; (d)), or up the Sacramento and San Joaquin Rivers (off the map in August 1996 and August 1997; (f, h)); in winter, it was located in Grizzly and Honker bays [February 1996; (e)] and in San Pablo Bay (February 1997 and January–February 1998; (g, i)).
The last comprehensive surveys of foraminiferal distributions and abundances in San Francisco Bay occurred in January and March 2010 (Fig. 11j; McGann, 2024; McGann et al., 2013, 2024). In total, 166 sites were sampled, and T. hadai continued to be a significant foraminiferal constituent based on those samples. Although abundances of the species appeared somewhat decreased as compared to the results from the bi-annual SFEI sampling in 1995–1998 (McGann, 2024), it should be noted that no samples were taken in eastern Central Bay where the previous surveys found abundances > 70 %. Therefore, the decrease may be misleading compared to those earlier years. In 2010, T. hadai still comprised 72 % and 81 % of the assemblages at two sites in South Bay. In the summers of 2014–2016, three samples were collected in northern South Bay (off Alameda and in San Leandro Bay) and Central Bay (off Point Isabel) with reported abundances of 21 %–50 % (Fig. 11k), whereas the abundances of T. hadai in two core top samples collected in November 2024 from Alameda and Point Isabel (cores BC01 and BC02) decreased to 9.6 %–14.2 % (Fig. 11l; McGann, 2024).
The 1930 to 2024 time series shows that the abundance of T. hadai changed dramatically in South Bay within 6 years (1980–1986), from a single location to nearly bay-wide in extent and from being very rare (1.5 %; Fig. 10e) to over 70 % of the fauna in some locations (Fig. 11a). A similar pattern of rapid expansion is commonly observed after a successful invasion by foraminifera (McGann et al., 2012; Eichler et al., 2018; Bouchet et al., 2023) and macroinvertebrates (Nalepa and Schloesser, 1993; Carlton et al., 1990; Nichols et al., 1990; Vinogradov et al., 1989; Buttermore et al., 1994), although other studies have shown lag periods between inoculation and spread of 30 years or more (e.g., in the Mediterranean Sea; Guastella et al., 2021). Trochammina hadai was equally widespread and abundant in 1987 and 1993 (Fig. 11b–c). A period of 8 years later and continuing for the following 4 years (1995–1998), it is evident that T. hadai had successfully invaded Central and North bays, again with dominant abundances (commonly > 30 % throughout) and > 70 % of the fauna in eastern Central Bay (Fig. 11d–i). It should be noted, however, that the spread into Central and North bays could have occurred far earlier than 1995, but a lack of samples in these locations from 1983–1994 makes this difficult to determine. Finally, the 2010 sampling effort throughout San Francisco Bay demonstrates that T. hadai was still geographically widespread (Fig. 11j) yet generally occurred in lower abundances than those observed 13 years earlier (1997). This pattern may be due to several factors: (1) the omission of samples from eastern Central Bay, (2) a seasonal effect due to the samples being collected only in the first quarter of the year, (3) highly brackish conditions caused by the unusually large amount of rainfall that year (ranked 28th highest out of the last 174 years; Golden Gate Weather Services, 2024), and (4) competition from other species of benthic foraminifera. Additional samples collected in 2014–2016 and 2024 in northern South Bay and Central Bay (Fig. 11k and l) support the hypothesis of a seasonal effect, as samples collected in the summer and winter in similar locations differ by ∼ 7 %–45 % (McGann, 2024), although a decrease in abundance of T. hadai due to competition or other factors over the intervening decade cannot be ruled out.
Cores BC01 and BC02 were taken in an attempt to further refine the invasion chronology illustrated by the time series. Core BC02 taken off Point Isabel in Central Bay was the more highly bioturbated of the two, as evidenced by the core photograph and X-Ray/CT scans (Fig. 8a, b, c) and the presence of worm tubes recovered during the sample processing throughout much of the upper 50 cm of the core. In this core, T. hadai was recovered in nearly every sample down to 47.0 cm (Fig. 8f), well below the Cs-137 first appearance datum of 1954 at 20.5 cm, and at an estimated age of ∼ 1740–1790 using a sedimentation rate of 1.64 mm yr−1. We assume that if the species was introduced somewhere in San Francisco Bay more than two centuries ago, it would have been found off Point Isabel prior to 1995 (Fig. 11d) when we have a definitive record of its first presence at that location. Instead, recovery of the species in sediments dated as 18th century is not reasonable and is attributed to downcore displacement by bioturbation, which is known to introduce error in both radiochemistry (Santschi et al., 2001) and biological records.
Core BC01 recovered off Alameda displays extensive shell material and little evidence of bioturbation in the core photograph and X-Ray/CT scans (Fig. 7a, b, c). The species was found down to a depth of 31 cm (Fig. 7f), below the Cs-137 maximum appearance datum of 1963 at 25.5 cm (Fig. 7e), and at an estimated age of 1950 using a sedimentation rate of 4.18 mm yr−1. Although less downcore displacement is evident in this core than BC02, T. hadai was not recovered in extensive surface sampling in this region in 1961–1972 (Fig. 10b, c), so this core also does not improve our understanding of the timing of introduction of T. hadai. It is possible that dated cores obtained in other locations may clarify when the species spread into other subembayments of San Francisco Bay.
4.3 Factors in the success of foraminiferal invaders
Trochammina is the oldest known multichambered foraminifer with a simple trochospiral test. The earliest well-preserved morphotypes of the genus are those described by Tremblin and Haig (2023) from the Lower Permian (Sakmarian; ∼ 294–290 Ma; Cohen et al., 2013) of the Irwin Basin in Western Australia in mudstone beds deposited in a very shallow-water and muddy estuarine-like interior sea. Tremblin and Haig noted that the Sakamarian species Trochammina geoffplayfordi Tremblin and Haig was a very close morphological analogue of modern T. hadai (Fig. 12), clearly demonstrating the conservative and slowly evolving nature of the genus. But does being one of the longest-lived foraminiferal genera contribute to its propensity to be a successful invader? Ammonia (e.g., Ammonia confertitesta) is another highly successful invader, yet its Neogene appearance is considerably younger (Loeblich and Tappan, 1988).
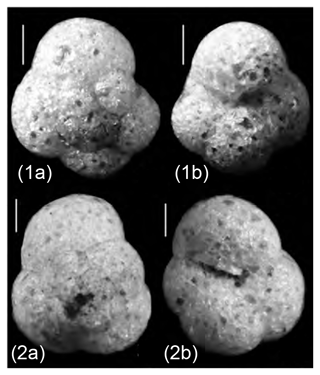
Figure 12Light microscope images. (1) Trochammina hadai from Leschenault Inlet, Australia; spiral side (1a), umbilical side (1b). (2) Trochammina geoffplayfordi (Sakmarian – Lower Permian; ∼ 294–290 Ma) acid-digested from a carbonate-cemented mudstone nodule, upper Holmwood Shale, Irwin Basin, Western Australia; spiral side (2a), umbilical side (2b). Photographs courtesy of Clément Tremblin and David Haig. Scale bars: 100 µm.
This question remains: what makes some foraminifera successful invaders and others not, even though they occur in the same environment? After Pianka (1970), k-strategists are conservative species with larger body sizes, longer life cycles, and essentially constant population sizes over time; they live close to the environment's carrying capacity. On the other hand, r-strategist species are known for their opportunistic behavior, small size, short life cycles, and highly variable population sizes without adjustment to available resources, mostly space and food. Many foraminifers display opportunistic behavior (Sjoerdsma and Van der Zwaan, 1992; Gooday, 1993; Alve, 1994). The genus Trochammina is known to be opportunistic (Reolid et al., 2014), epifaunal or infaunal depending on the species, and characterized by a diverse feeding strategy (passive herbivore, grazing herbivore, detritivore, omnivore, and bacterivore; Koutsoukos and Hart, 1990). As a result, Trochammina species can survive in and adapt to unfavorable environmental conditions, including those that are nutrient-enriched and oxygen-limited.
In a study of Lower Jurassic deposits from northern Siberia, a benthic biotic crisis (mass extinction) took place in response to the Toarcian Oceanic Anoxic Event, which was the result of high total organic carbon (TOC) and a severe decrease in oxygen (Reolid et al., 2014). The diversity of the benthic foraminiferal assemblage dropped significantly and was dominated (42 %–91 % of the fauna) by the agglutinated taxon Trochammina. In other Upper Triassic to Upper Jurassic studies, low-diversity, Trochammina-dominated agglutinated assemblages were widespread in Austria (Golebiowski, 1990; Kuerschner et al., 2007; Clémence et al., 2010), Norway (Nagy and Berg, 2008; Nagy et al., 1990; Reolid et al., 2010), the Barents Sea (Bremer et al., 2003; Nagy et al., 2010), and the northern North Sea, Yorkshire, and north Scotland, UK (Nagy et al., 2010). Trochamminids were also among the most robust species persisting for almost 100 years of high organic matter discharge from a pulp and paper mill in a highly polluted Swedish–Norwegian fjord (Polovodova Asteman et al., 2015). It appears that being an opportunist allows a species to successfully colonize new environments.
The ability to adapt to stressed environments applies to T. hadai as well. The species is known to flourish in a wide range of water temperatures and environmental conditions such as low oxygen and organic pollution (Kitazato and Matsushita, 1996; Tsujimoto et al, 2006; Lee et al., 2016a, b; Eichler et al., 2018; McGann et al., 2019a; Eichler, 2024). In contrast to this however, Lesen (2005) found that while the standing stock of T. hadai peaked in two successive springs (2000 and 2001) in South Bay when water column chlorophyll and sediment TOC levels peaked during the spring bloom period, the standing stock then decreased the following fall (2001) despite the fact that the sediment TOC level was the highest. She concluded that this pattern suggests the species may be outcompeted by other species (e.g., Fursenkoina pontoni (Cushman)) that can more quickly exploit blooms in production or reproduce rapidly when environmental conditions become optimal for them. Trochammina hadai also has a biphasic lifestyle (Matsushita and Kitazato, 1990; Kitazato and Matsushita, 1996), with the production of free-swimming gametes in its sexual phase enhancing dispersal (Ruiz et al., 2000).
4.4 Global occurrences of Trochammina hadai
We compared indigenous Japanese specimens of T. hadai with introduced specimens from habitats along the US Pacific coast and other locations globally. There are very few differences between 18S sequences derived from T. hadai and specimens sequenced from Japan (Hamana Lake), Western Australia (Leschenault Inlet), the US Pacific coast (Padilla Bay, Humboldt Bay, San Francisco, Santa Barbara, and Los Angeles), and Europe (Le Havre, France, and Gothenburg, Sweden), which all cluster in a well-supported group. Therefore, both the molecular and morphological data suggest they are the same species of T. hadai (Fig. 5; Plates 1–4).
To evaluate the genetic diversity within T. hadai, we analyzed complete ITS rDNA for 17 individuals sampled from three different regions in Japan (Hamana Lake, Akkeshi Lake, and Matsushima Bay) and five locations along the US Pacific coast (Samish Bay, San Francisco Bay, Santa Barbara, Los Angeles, and San Diego; Fig. 6). The ITS tree is divided into two groups that lack BV support. Genetic differences within the ITS region are due to short or clustered indels and substitutions. Pairwise genetic distance within individuals ranges from 0.003 to 0.020 and is close to pairwise genetic distance between individuals that ranges from 0.002 to 0.035. The lack of genetic difference indicates that there is no cryptic speciation among the collected specimens and the T. hadai specimens in this study are part of a big homogenous population. However, ITS data have been obtained only for few specimens from Japan and the US Pacific coast, and further analyses are needed to determine the magnitude, origin, and direction of genetic exchange between local populations in different geographic regions.
From morphological and molecular analyses, we conclude that T. hadai has been documented in 77 estuaries or harbors along the western coast of North America (73 in the USA and four in Canada; Fig. 4b–d; Table S1; Plate 2, Figs. 2a–b; Plate 4, Figs. 1–16) but has not yet been recorded in the Hawaiian Islands. The absence in 2022 of this species at sites where it occurred in previous surveys does not necessarily mean that T. hadai no longer resides there but reflects the fact that new sampling sites at those locations were investigated. Trochammina hadai has also been recovered at nine sites in Sweden, two in France, three in Brazil, and two locations at one site in Australia (Fig. 13; Plate 2, Figs. 3a–5b; Plate 3, Figs. 3a–5b). Specimens from Brazil in Flamengo Inlet (Eichler et al., 2018), in anoxic Florianópolis Bay sampled in 2014 (Eichler, 2024), and Paranaguá Harbor (as Portatrochammina sp. in Pupo and Disaró, 2006 and Ammoglobigerina globigeriniformis Parker and Jones in Faria et al., 2021) have not yet been sequenced but have sufficiently similar morphology to be considered T. hadai as well (Plate 2, Fig. 6a–b; Plate 3, Figs. 6a–8b).
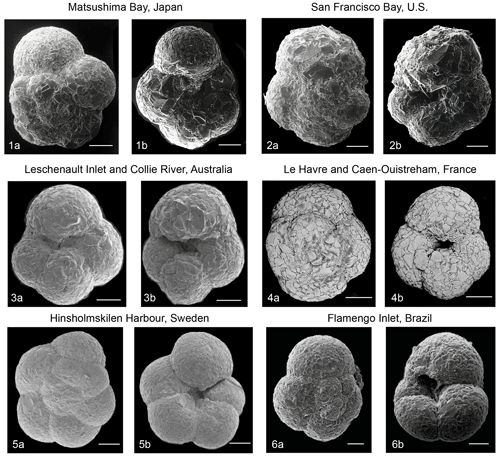
Plate 2Scanning electron micrographs (SEMs) of selected Trochammina hadai specimens worldwide. (1) Matsushima Bay, Japan; spiral side (1a), umbilical side (1b). (2) San Francisco Bay, USA; spiral side (2a), umbilical side (2b). (3) Leschenault Inlet and Collie River, Australia; umbilical side (2a), umbilical side (2b). (4) Le Havre and Caen-Ouistreham, France; spiral side (4a), umbilical side (4b). (5) Hinsholmskilen Harbour, Sweden; spiral side (5a), umbilical side (5b). (6) Flamengo Inlet, Brazil; spiral side (5a), umbilical side (5b). Photographs courtesy of Masashi Tsuchiya (1, 2), Clément Tremblin (3), Jean-Charles Pavard (4), Irina Polovodova Asteman (5), and Patrícia Eichler and André Rodrigues (6). Scale bars: 100 µm.
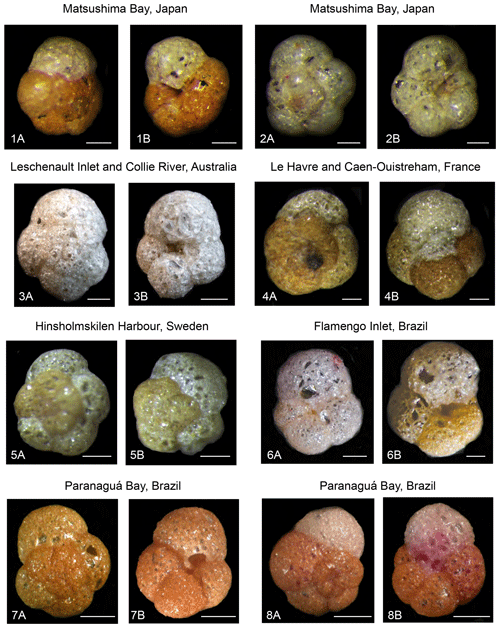
Plate 3Light microscope images of Trochammina hadai from global sites. (1, 2) Matsushima Bay, Japan; spiral side (1a, 2a), umbilical side (1b, 2b). (3) Leschenault Inlet and Collie River, Australia; spiral side (3a), umbilical side (3b). (4) Le Havre and Caen-Ouistreham, France; spiral side (4a), umbilical side (4b). (5) Hinsholmskilen Harbour, Sweden; spiral side (5a), spiral side (5b). (6) Flamengo Inlet, Brazil; umbilical side (6a), umbilical side (6b). (7–8) Paranaguá Bay, Brazil; spiral side (7a, 8a), umbilical side (7b, 8b). Photographs courtesy of Masashi Tsuchiya (1, 2), Clément Tremblin (3), Jean-Charles Pavard (4), Irina Polovodova Asteman (5), Patrícia Eichler and André Rodrigues (6), and Sibelle Trevisan Disaró (7, 8). Scale bars: 100 µm.

Plate 4Light microscope images of Trochammina hadai from coastal western North America. (1) Nanaimo Harbour. (2) Davis Lagoon. (3) Padilla Bay, Washington. (4) Willapa Bay, Washington. (5) Coos Bay, Oregon. (6) Humboldt Bay, California. (7) Bodega Bay, California. (8) Tomales Bay, California. (9) San Francisco Bay, California. (10) Morro Bay, California. (11) Santa Barbara, California. (12) Marina Del Rey, California. (13) Los Angeles/Long Beach Harbor, California. (14) Newport Beach, California. (15) Mission Bay, California. (16) San Diego Bay, California. Sites (1)–(2) in British Columbia, Canada; sites (3)–(16) in the USA. All views are of the umbilical side, except Coos Bay which is a twinned specimen. Photographs courtesy of Mary McGann (1–2, 4–12, 14–16) and Maria Holzmann (3, 13). Scale bars: 100 µm.
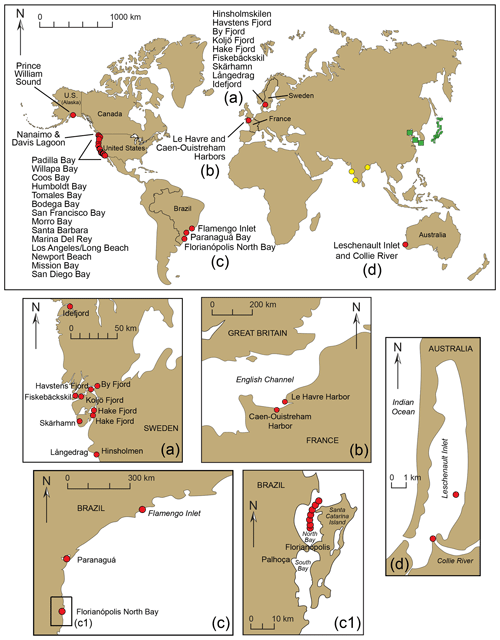
Figure 13Worldwide locations of foraminiferal studies reporting the presence of Trochammina hadai. Red circles indicate where the species is non-indigenous, green squares indicate where the species is native, and yellow circles indicate where the species is suspected of being an NIS. (a–d) Detail of sites in Sweden, France, Brazil, and Australia, respectively. Panel (a) is modified from Pavard (unpublished data); panel (b) is modified from Bouchet et al. (2023); panels (c) and (c1) are modified from Eichler et al. (2018) and Eichler (2024); and panel (d) is modified from Tremblin et al. (2021). Panel (c1) shows panel (c) in more detail, showing the sites in Florianópolis where T. hadai has been found.
In Sweden, T. hadai has been found preferentially residing in small boat harbors of the city of Gothenburg, such as Hinsholmskilen Harbour (Polovodova Asteman et al., 2025) and Långedrag (Jean-Charles Pavard, University of Gothenburg, unpublished data, 2024); in two other harbors of the west Swedish coastline, i.e., Skärhamn (Asplund, 2024) and Fiskebäckskil (Pavard, unpublished); and in shallow fjords (Ide, Havstens, By, Koljö, and Hake fjords) on the Swedish west coast based on metabarcoding (Brinkmann et al., 2023; O'Brien et al., 2024, 2025) and morphological data (Axelsson, 2024) (Fig. 13a). At Hinsholmskilen Harbour, the species was a NIS in 70 % of the samples studied in the harbor and comprised 23 % of the assemblage at one station located in the middle harbor (Polovodova Asteman et al., 2025). In samples from a sediment core from Skärhamn, Asplund (2024) found abundances of T. hadai ranging between 3.8 % and 26.3 %. The sample containing the highest abundance of the species also had plentiful organic material. In Hake Fjord, Axelsson (2024) found the abundance of T. hadai in a sediment core ranging between 0 % and 24 % from 0–21 cm downcore; the species was not present below 21 cm. In Fiskebäckskil, T. hadai represented about 12 % of the total assemblage and approximately 23 % in one replicate out of three, whereas in Långedrag, it was only a minor component (1 %–2 %) of the total assemblage (Pavard unpublished). Most of these Swedish settings are either prone to severe heavy metal pollution or severe hypoxia.
Elsewhere in Europe, T. hadai was identified based on morphological and molecular data along the coast of Normandy, mainly in Le Havre Harbor but also Caen-Ouistreham Harbor (Bouchet et al., 2023; Fig. 13b). Like in native habitats of Asia, the species was found in transitional waters with muddy sediments and exhibited high relative abundances (up to about 40 %), confirming that T. hadai is a highly competitive NIS in this location.
In Brazil, no specimens of T. hadai were found in studies conducted in the southeastern region of Ubatuba, São Paulo State, in the 1990s and 2000s (Duleba, 1994, and Duleba et al., 1999, in Flamengo Inlet; Burone and Pires-Vanin in Ubatuba Bay (1994); and Silva and Duleba, 2013, in Fortaleza Inlet in 2008), but four living specimens were recovered in Flamengo Inlet in 2010 (Rodrigues et al., 2014) (Fig. 13c, c1). The environment of Flamengo Inlet is greatly impacted by chronic and acute stress, particularly from domestic sewage, oil, and gasoline leaks and erosion (Sanches, 1992; Rodrigues et al., 2014). Organically enriched sediment had TOC values averaging 7 % but reaching up to 11 %. Just 4 to 5 years later (2014–2015), the species had become well established, occurring at more than two-thirds of the 18 sites studied (Eichler et al., 2018). Generally, the T. hadai abundances were low (< 4 %), but the species comprised 13 %–18 % of the assemblage at three locations. Trochammina hadai was also first recovered in 2005 in Paranaguá Harbor (Pupo and Disaró, 2006; Sibelle Trevisan Disaró, Federal University of Paraná, personal communication, 2018) and in samples collected in 2014 from Florianópolis North Bay. At the latter location, the sediments were oxygen depleted with high organic matter, which are favorable conditions for the occurrence of T. hadai. The species comprised 4.57 % and 3.19 % of the foraminiferal assemblages in May and October, respectively (Eichler, 2024; Fig. 13c, c1). These are further examples that, as in Japan and elsewhere, this NIS tolerates and even thrives in contaminated locales.
Leschenault Inlet and the associated lower Collie River, linked to Bunbury Port in southwestern Australia, is the only Australian location where T. hadai has been confirmed by molecular analysis (Tremblin et al., 2021; Fig. 13d). In the mud samples here, Tremblin et al. (2021) noted that T. hadai and Ammonia spp. were the dominant species, accompanied by species of Ammobaculites, Cornuspira, Elphidium, Nonion, and Quinqueloculina (also refer to Revets, 2000). In other isolated estuaries along over 2000 km of coastline in southwestern Australia, T. hadai has not been found.
4.5 Global vectors and spreading rates
4.5.1 West coast of North America
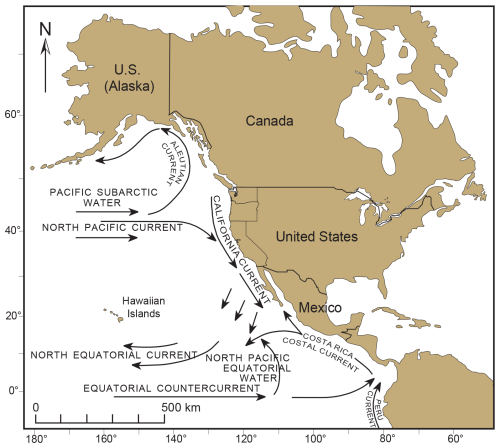
Our earliest record of T. hadai along the western US seaboard is in Puget Sound (McGann et al., 2012), specifically in Padilla Bay. How was the species introduced there? Since T. hadai is infaunal, not epibiotic, it is unlikely that it traveled from Asia to the west coast attached to marine plants or floating anthropogenic debris. Instead, could the dispersal of planktonic propagules (although not yet confirmed for T. hadai) passively carried by the prevailing eastern-flowing North Pacific Current across the Pacific Ocean (Fig. 14) have resulted in the introduction of T. hadai in Puget Sound? The speed of this current is 0.03–0.06 m s−1, and the distance from Japan to Puget Sound is ∼ 4000 nmi. Using the higher speed, it would take the propagules more than 23 months to make the journey across the Pacific. Most benthic foraminifera are known to have short life spans of from a few weeks or months to approximately a year in favorable environmental conditions (Cearreta, 1988; Lee et al., 1991; Langer, 1993; Murray, 1991). Examples of this are Ammonia (4–5 months; Cearreta, 1988), Haynesina germanica (3 months; Cearreta, 1988), and Planorbulina mediterranensis d'Orbigny (> 10 months; Langer, 1993). Foraminiferal life spans may also extend as environmental conditions deteriorate. Murray (1991) and Hayward et al. (2014) suggested they may live up to 5 years or even as much as 13 years in marsh environments with extreme conditions (Hayward et al., 2014). From a study in shallow, brackish Hamana Lake in Japan, Matsushita and Kitazato (1990) determined that the average life span of T. hadai was only 3–6 months. Therefore, it seems unlikely that living propagules, juveniles, or adults of T. hadai would survive the journey across the Pacific to successfully colonize Puget Sound or other west coast sites of the USA. The same would be true of transits across the Atlantic Ocean to Europe or Brazilian destinations, as well as across the Pacific or Indian oceans to Australia. It should be noted, however, that some species may use “stepping stones” to migrate over vast distances (McGann et al., 2019b), or their propagules can go into a dormant state under certain conditions, thereby lengthening the overall life span significantly (Ross and Hallock, 2016), which might also facilitate longer dispersal routes.
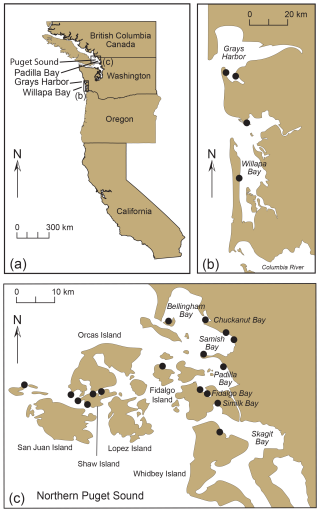
Figure 15(a) Map of the west coast of the USA with selected sites of early (1850s) oyster culturing. (b–c) Location (black circles) of historic Olympia oyster beds in (b) Grays Harbor and Willapa Bay and (c) Northern Puget Sound. Panel (c) modified from Dinnel et al. (2009).
Padilla Bay was one of the sites of early oyster farming along the west coast (Fig. 15a, b). Historical oyster beds were located in other Puget Sound locations as well, including Bellingham, Chuckanut, Samish, Fidalgo, and Similk bays; Orcas and Shaw islands; and the northeastern shore of Whidbey Island (Fig. 15c; Dinnel et al., 2009). Small, native oysters (Olympia oysters, Ostrea lurida Carpenter) were cultivated commercially in Samish and Willapa bays in the early 1850s and became highly sought after in San Francisco due to the California gold rush of 1848–1855 (Caswell, 2016). Due to their natural slow growth and accelerated harvesting, supplies began to diminish in the 1870s, so oysters from Chesapeake Bay in the eastern USA (Eastern or Atlantic oyster, Ostrea virginica Gmelin) were transported to Willapa Bay in 1896 (Esveldt, 1948). In 1902 and 1903, Japanese entrepreneurs brought Hiroshima oysters to Samish Bay from Kobe (Hyōgo Prefecture), and in 1906–1907, Chiba oysters from Tokyo Bay were planted in several locations, including Willapa Bay, but these transplant efforts were not successful (Juzo Hori, History of transplantation of Japanese oysters to the United States, Tokyo Imperial Fisheries College, unpublished, 1947; Esveldt, 1948; Steele, 1964; Hiroshi Kitazato, Tokyo University of Marine Science and Technology, personal communication, 2024). Instead, the Eastern oyster continued to be cultivated until an unexpected die-off occurred in 1919. The die-off allowed for the introduction of the large Pacific oyster (Crassostrea gigas (Thunberg)) from Miyagi Prefecture, Japan, to Samish and Willapa bays shortly thereafter, as well as in Grays Harbor in 1933 (Fig. 15b) (Juzo Hori, History of transplantation of Japanese oysters to the United States, Tokyo Imperial Fisheries College, unpublished, 1947; Esveldt, 1948; Steele, 1964; Hiroshi Kitazato, Tokyo University of Marine Science and Technology, personal communication, 2024).
Similarly, in southwestern British Columbia, Canada, Pacific oysters were first introduced in Ladysmith Harbour and Fanny Bay (located 23 km south and 82 km north of Nanaimo, respectively) in 1912–1913 from stock sent from Miyagi Prefecture (Lavoie, 2019). Because many of the adult Pacific oysters did not survive the 2-week transit from Japan, importation of seed oysters was started instead. The oyster shells with attached spat (referred to as “sets”) were first reported in Ladysmith Harbour in 1925 (Lavoie, 2019). In 1926, 2000 oysters were transplanted to the harbor from Samish Bay as well as seed oysters from Japan, and by 1929–1932, 4 million seed oysters were imported from Japan (Lavoie, 2019). In both British Columbia and Puget Sound, the seed oysters and marine plants in which they were packed and transported, as well as the associated sediment, were dumped directly into the local waters. The larvae were allowed to grow and were then harvested. It was in this packing material and sediment (as discussed above) that specimens of T. hadai could have easily been introduced into those bays and harbors from Japan. Possibly the NIS Ammonia confertitesta arrived in the Ladysmith Harbour area around this time as well (McGann and Holzmann, 2024). Furthermore, oyster farming produces lots of feces, resulting in organic rich sediment under the farms, which may cause a collapse in the natural nutrient cycles. In turn, dysoxic or even anoxic bottom conditions may develop, and T. hadai is known to thrive in dysoxic environments.
Although we cannot definitively determine the timing or location of T. hadai's introduction along the western US seaboard, a core from Padilla Bay suggests the species arrived there at some point between the late 1800s and 1971 (McGann et al., 2012). We tentatively assume the NIS arrived in Samish, Padilla, and Willapa bays with the oyster transplants from Japan from 1902 to the 1920s and in southwestern British Columbia (Ladysmith Harbour) in the 1920s. From these locations, the dispersal of propagules carried by the prevailing currents could have easily dispersed the NIS the short distances throughout other bays and harbors of northern Puget Sound and the San Juan Islands (Fig. 15c). It is less certain that they could reach other ports and harbors along the west coast of the USA besides those of Puget Sound. The California Current flows southward along the coast (Fig. 14) at speeds of 5–10 cm s−1, and the distances between most ports and marinas are large: Puget Sound (Seattle) to San Francisco Bay (931 nmi), San Francisco to Los Angeles/Long Beach (475 nmi), and Los Angeles to San Diego (55 nmi). At the higher speed, it would take a foraminifera 6.7 months to reach San Francisco Bay from Puget Sound. This is pushing the limit of the life span of T. hadai, although as already discussed, if the species produced dormant propagules, the life span could be much longer.
Fishing boats and pleasure boats carrying foraminifera on their anchors and anchor chains is clearly a means by which specimens of T. hadai may be transported between all ports and marinas along the west coast of the USA that are too small for large transoceanic vessel traffic; in our study, this includes Bodega, Tomales, Morro, and Mission bays; Santa Barbara Harbor; Marina Del Rey; and Newport Beach (Fig. 4). However, the amount of sediment deposited in those locations by this vector would not be large, diminishing the chance of viable introductions. Instead, transport of foraminifera in ballast tanks is a means by which several billion metric tons of water worldwide (David et al., 2015) and tens of tons to 200 t of sediment per vessel (Drake et al., 2005; Johengen et al., 2005) are carried between ports with a much higher potential for introductions upon their release.
The advent of shipping commercial products on a global scale expanded greatly after World War II and into the mid-20th century, including both bulk carriers and tankers, which utilized ballast tanks for stability. Transit times have decreased over the years, so now it only takes a few weeks to travel across vast oceans between ports (SEARATES, 2019). With the short transit times, foraminifera can easily remain viable during the trip. Therefore, T. hadai, with a life span of 3–6 months or possibly longer if conditions are poor, should have no problem surviving transits between global ports in ballast tanks.
According to Seebens et al. (2013), the most vulnerable areas to marine bio-invasions by means of commercial shipping include the northwest Pacific, Mediterranean Sea, central Indo-Pacific, and northwest Atlantic. Trade routes with a high invasion probability include the USA to Scandinavia and Brazil to the Mediterranean Sea; low invasion probability routes span Asia to Brazil, Asia to Australia, France to Australia, the USA to Brazil, and the Panama Canal to Scandinavia and France (Seebens et al., 2013; Fig. 16). In the northwest Pacific, Asia is the primary source of NIS introductions, and there is a high probability of invasion between those trading partners (Seebens et al., 2013; Fig. 16). We have surmised that T. hadai arrived in the plant material and sediment associated with oyster and larvae transported to Puget Sound from Japan in 1902 to the 1920s, but we also assume that the species was introduced to the west coast of the USA through not a single but repeated inoculations through the years, whether through Japanese oysters or ballast water and sediment. The major ports on the western US seaboard in our study that are frequented by bulk carriers and tankers, and therefore could have ballast water and sediment as a vector of introduction, include Prince William Sound, Nanaimo, Bellingham Bay in Puget Sound, Humboldt Bay, San Francisco Bay (i.e., the Port of Oakland), Los Angeles/Long Beach ports, and San Diego Bay (Fig. 4).
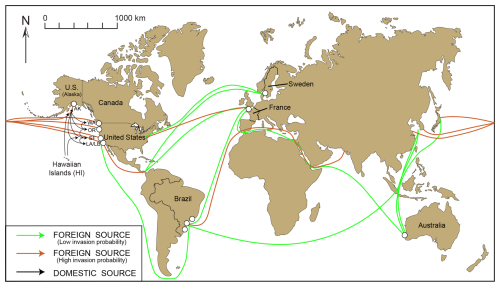
Figure 16Simplified map (modified from Seebens et al., 2013) of invasion probability of trade routes to the sites where Trochammina hadai has been recovered in the USA, Sweden, France, Brazil, and Australia. Black trade routes represent a domestic source, green trade routes a foreign source with low invasion probability, and red trade routes a foreign source with a high invasion probability. The invasion probability (i.e., total invasion risk) is based on the study by Seebens et al. (2013) of 717 250 ballast water releases from 1 January 2004 to 12 July 2012, with each species passing three distinct and independent invasion transitions: (1) the probability that a species is alien in a recipient port; (2) the probability of the species' introduction (i.e., it survives being entrained in ballast tanks), which decreases with travel time and increases with the amount of released ballast water; and (3) the probability of the species' establishment in the recipient port, which increases when donor and recipient ports have similar environments in terms of water temperature and salinity. San Francisco Bay was one of the four highly invaded sites used in their study. The US domestic ports along the northwestern Pacific seaboard are HI (Hawaii), LA/LB (Los Angeles/Long Beach ports), SF (San Francisco Bay, California; Port of Oakland), OR (Portland, Oregon), WA (Puget Sound, Washington), and AK (Port Valdez, Prince William Sound, Alaska).
Today, the Port of Oakland ranks among the four largest Pacific coast ports for container cargo, along with Los Angeles and Long Beach in southern California and Seattle and Tacoma in Washington state. In 2023, about 74 % of the Port of Oakland's trade was with Asia, 17 % with Europe, 3 % with Australia or Aotearoa / New Zealand and other Pacific islands, and about 1 % with other foreign nations; about 5 % was domestic (primarily Hawaii) (Oakland Seaport, 2024). Similarly, the top five foreign trade routes of the Port of Los Angeles in 2023 were Northeast Asia (61 %), Southeast Asia (27 %), India Sub-continent (3 %), Northern Europe (3 %), and Mediterranean (2 %) (The Port of Los Angeles, 2024). The Port of Long Beach's trade is more than 90 % East Asian, and the five top foreign trading partners are China, Vietnam, Thailand, South Korea, and Taiwan (Port of Long Beach, 2024). Smaller trading partners are Hong Kong SAR, Indonesia, Malaysia, Italy, Cambodia, Australia, and Japan. The top five trading partners for the combined ports of Tacoma and Seattle include China/Hong Kong SAR, Japan, Alaska, South Korea, and Taiwan (City of Tacoma, 2024) and, for the Port of San Diego in 2019, Japan, Germany, and South Korea (California Association of Port Authorities, 2024). Clearly, Asia is the primary source of trade in coastal northeastern Pacific ports.
San Francisco Bay is one of the few locations where we may get an approximation of a spreading rate due to the repeated surface sampling that occurred there over several years. The last comprehensive bay-wide survey occurred in 1961–1970 (Fig. 10b) through the studies of Conomos (1963), Means (1965), Slater (1965), Gram (1966), Quinterno (1968), and Locke (1971). The only region not covered in these studies was Central Bay, which was undertaken in 1972 by Wagner (1978) but which was only partial coverage of that subembayment (Fig. 10c). None of these studies reported the occurrence of T. hadai. The species was also not recovered in subsequent sampling based on samples collected by Nichols, Sloan, and Brunner in 1980-1981 (Fig. 10d; Doris Sloan, University of California Berkeley, personal communication, 1997; McGann et al., 2024) until the single sample in 1983 from South Bay (Fig. 10e). We have already discussed how quickly the NIS became a dominant constituent of the foraminiferal assemblage. This is demonstrated in Fig. 11a. The open circles represent sites where no specimens of T. hadai were found in 1980–1983. Overlying this is the abundance of the species in 1986. In just 6 years, T. hadai dominated the assemblage at six of the nine sites. Similarly, the three sites near the eastern edge of Central Bay where the species was not found in 1980–1981, had abundances of > 70 % a maximum of 15 years later (August 1995, Fig. 11d). The invasion could have occurred even more quickly, but we do not have the samples to confirm this. Our limited sampling also allows us to determine that the species spread throughout San Francisco Bay in a maximum of 34 years (1961–1995; compare Figs. 10d and 11d) but possibly much sooner.
4.5.2 France
In the 1700 and early 1800s, native flat oysters (Ostrea edulis Linnaeus) were obtained by dredging off boats in France. In 1858, the first spat collection was undertaken, and in 1859, the first oyster beds were created (Arcachon Bay, 2024). Oyster farming expanded in France between 1870 and 1890 but collapsed due to marketing problems in 1891–1896. In 1920, the native oyster population was decimated by a viral disease, and cultivation of the Portuguese oyster (Magallana angulata (Lamarck)) began mainly in Marennes-Oléron Bay and Arcachon Bay but also in the Bay of Bourgneuf, La Rochelle, and Gironde Estuary, only to again succumb to a viral disease in 1970–1971 (Gizel and Héral, 1991). Probably around 1966, large Pacific oysters (Crassostrea gigas) and their spat were imported from Japan in Marennes-Oléron Bay (Le Borgne et al., 1973), where they have continued to be cultivated. All four of these sites are situated in the Bay of Biscay. The Port of La Rochelle is the most northern of the four and lies about 1100 km southwest of the Port of Le Havre. Although the prevailing tides flow northward from the Bay of Biscay and then eastward in the English Channel, the distance seems too great to expect any propagules of T. hadai to survive and arrive in sufficient numbers to establish a population there. Instead, Le Havre Harbor holds a key geographical position, being the first port along the Northern Range (i.e., one of the busiest port ranges in the world from Le Havre to Hamburg, also including Antwerp and Rotterdam) where ships can stop when entering Europe. The Northern Range is the main shipping interface between Europe and Asia, making Le Havre the shortest international shipping route from Asia to Europe. No less than two-thirds of the international shipping entering Le Havre Harbor is from Asia, and international trade between these two places dates back to the 19th century, with a sharp increase in the early 1990s. In Normandy, T. hadai was only very abundant in heavily modified habitats like harbors, suggesting that ballast water and sediment likely constitute the vector of introduction. However, it was nearly absent from natural transitional waters suggesting that the propagation outside the Normandy harbors may be limited in the future.
4.5.3 Sweden
Some locations where T. hadai has been observed as an NIS in Sweden are small boat harbors (i.e., Fiskebäckskil, Skärhamn, Långedrag, and Hinsholmskilen Harbour), in which the vector of introduction is likely mud on anchors and anchor chains of pleasure and fishing boats. For sites located in the shallow west coast fjords, the means of introduction is more complex. Though there are a lot of marinas around, the fjords are also located on plausible shipping routes to some bigger harbors such as Halden (Norway) for the Idefjord; Udevalla for Koljö, Havstens, and By fjords; or both Wallhamn and Stenungsund harbors for the Hake Fjord. All these are not the biggest harbors along the west coast of Sweden, but they still host some substantial industries and bigger ships/vessels, in addition to hosting small boat harbors. These bigger harbors could also be other first introduction spots, and then the pleasure boat traffic could help spread T. hadai or other NIS foraminifera into the fjords. This could also be the case for the small boat harbors of Långedrag and Hinsholmskilen Harbour, located at the end of the mouth of the Göta River estuary, which hosts the largest international trade harbor in Scandinavia, the Port of Gothenburg and marinas, or for the Fiskebäckskil small boat harbor, located next to the town of Lysekil, which also hosts a large harbor with some industries. As such, this would well represent the “bridgehead effect” (Bertelsmeier et al., 2018), with the Port of Gothenburg and/or smaller industrial harbors being the first introduction spots around and then small industrial/small boat harbors being secondary ones.
Based on preliminary sediment core studies, the arrival of T. hadai to Sweden may have taken place sometime after the 1970s (Axelsson, 2024). The timing of the species' arrival suggests the possibility that oysters from Japan (and their packing material) were the vector of introduction. Originally, the native flat oyster Ostrea edulis was cultivated in Sweden. With the viral-induced oyster collapse that decimated both the native (1920) and Portuguese oysters (1970–1971), the Pacific oyster Crassostrea gigas was introduced from Japan to Europe in the 1960s (Andrews, 1980; Drinkwaard, 1999) and to Scandinavia for aquaculture trials in the 1970s (Wrange et al., 2010). This did not cause an establishment of wild populations until the 1990s when the Pacific oysters were observed in the Danish Wadden Sea (Diederich et al., 2005). The main invasion event in Sweden, though, first occurred in 2006/2007 (Wrange et al., 2010; Strand et al., 2012; Greeve, 2023); C. gigas has now completely taken over the shallow west coast bays (Strand et al., 2012; Greeve, 2023). Denmark's shellfish hotspot, the Limfjord in North Denmark, may have been a primary vector location. Based on this, the introduction of T. hadai to Sweden may have occurred in connection with the first aquaculture trials with C. gigas in the 1970s.
4.5.4 Brazil
Pacific oyster larvae were first introduced into Brazil in 1974 from Great Britain and into Cananéia, São Paulo, from Japan in 1975 (Akaboshi, 1979; Akaboshi et al., 1983). Brazil's main oyster producer is the state of Santa Catarina (91 %; Oliveira Neto, 2008), with most cultivation occurring in the North and South bays of Florianópolis Island and around Palhoça (Melo et al., 2010; Fig. 13c, c1). Because no commercial shipping occurs in the North Bay of Florianópolis, we suggest the T. hadai present there (Eichler, 2024) was introduced to the North Bay of Florianópolis in the marine plant matter used for packing when the shipment of Pacific oysters arrived from Japan. Flamengo Inlet, just south of Ubatuba, also has no commercial shipping traffic, so it seems likely that the species spread there from Florianópolis in mud on anchors and anchor chains of active commercial fishing and recreational boating traffic. There are also four major ports (i.e., Santos, São Sebastião, Angra dos Reis, and Itaguaí) within ∼ 250 km north and south of Ubatuba (Oliveira, 2008) that could have been potential sources of T. hadai through ballast water and sediment releases. Similarly, a large port city, Itajai, is situated just 100 km north of Florianópolis and may also be a source of T. hadai. Paranaguá Harbor, situated approximately ∼ 700 km southwest of Flamengo Inlet, is strikingly different from the other two locales by being a major coastal port city with extensive commercial shipping. Ballast water and sediment releases occur regularly, and numerous alien micro- and macroinvertebrate species have been introduced there (refer to Oliveira, 2008, and references therein). For example, an outbreak of cholera in Paranaguá in 1999 was attributed to ballast water discharges (Oliveira, 2008). We surmise that the dumping of marine plant matter associated with the introduction of Pacific oysters from Japan in 1975 was a primary vector introducing T. hadai into southern Brazil, as well as the release of ballast water and sediment, obtained at distant locations, into the ports of Paranaguá, Santos, São Sebastião, Angra dos Reis, Itaguaí, and Itajai, with a secondary vector being the transport by fishing boats and pleasure boats to the smaller marinas.
The only introduction timeline we have for T. hadai in Brazil is in Ubatuba in the 1990s and 2000s where the species was absent in Flamengo Inlet (Duleba, 1994 and Duleba et al., 1999), Ubatuba Bay (Burone and Pires-Vanin, 1994), and Fortaleza Inlet in 2008 (Silva and Duleba, 2013) and then appeared very rarely in Flamengo Inlet in 2010 (present but not statistically significant at only 2 of 34 sites; Rodrigues et al., 2014). By 2014–2015, the species had become established at 14 of the 18 sites studied (< 4 % at seven sites, 13 %–18 % at three more sites, and present at four more sites with statistically insignificant counts; Eichler et al., 2018). We assume this rapid, 5-year introduction chronology is another example of the “Bridgehead” effect, where species are introduced by a primary vector (the release of oysters with marine plant matter and associated sediment or ballast water and sediment in this case) elsewhere and then are carried to the site by secondary vectors (anchors of fishing and pleasure boats).
4.5.5 Australia
Unlike France, Sweden, or Brazil, oyster culturing does not appear to have been a vector for the introduction of T. hadai in Leschenault Inlet, Australia. Although Pacific oysters were successfully introduced from Japan to western Australia in 1947, they eventually died out (NSW Government, 2024). In the 1980s, the oysters entered New South Wales waters, invading many intertidal regions and coastal waterways. Due to the great distances (2680 km) between these sites though, we assume the release of oyster larvae and transport in currents was not a viable vector of introduction of T. hadai into Leschenault Inlet. Instead, Bunbury Port, located only 13.5 km south of Leschenault Inlet, is a major wood chip exporting site in southwestern Australia with most of the commodity destined for Japan. Transported via small vessels with shallow drafts allowing navigation at water depths of around 20 m, these vessels release ballast water (including mud) before they take on the wood chip load. Tremblin et al. (2021) argued that T. hadai was introduced in Leschenault Inlet via ballast water from Japan released when the ships were in or near the port. It is also possible that transport on the anchors and anchor chains of pleasure or fishing boats, or by planktonic propagules carried on local currents, could be responsible for the introduction of T. hadai over the short distance. However, we only have surface samples at this location and therefore cannot determine a spreading rate.
Further investigations, particularly with dated cores, could help refine the spreading rates at these global sites. We must also be aware that invasions occur in both directions between trading partners. An example of this was the discovery by one of us (Hiroshi Kitazato) of Ammonia lineages originating from coastal California sediments on the Kanazawa Hakkei coast, which is located near the US Naval base at Yokosuka, Japan. It appears this lineage group invaded Japan from the USA after World War II, most likely through the release of ballast water and sediment from the naval ships.
Seebens et al. (2017) identified three major patterns of non-native species introductions globally:
-
Pattern 1: for algae, invertebrates, and some vertebrates there were weak increases until ∼ 1950, followed by strong increases via the unintended consequences of increasing trade;
-
Pattern 2: a decrease in the deliberate translocation of mammals and fish after ∼ 1950;
-
Pattern 3: a high rate of introductions of plants in the 19th century associated with European colonization, with a continued high rate in the latter part of the 20th century associated with trade (e.g., of food, pets, and ornamental plants).
At many of the global sites where T. hadai has been found, ballast water and sediment appear to be a primary vector of introduction of the NIS in the major ports, so pattern 1 clearly illustrates the global spread of T. hadai. The species' rapid spread globally is also likely the best example of a protist for identifying recently accumulating “Anthropocene” strata, and moreover its rapid intercontinental distribution dovetails with the post-World War II Great Acceleration sensu Steffen et al. (2015), for which international shipping is a key marker and which is the driving mechanism of the Anthropocene.
Natural migrations from Asia to the North American continent through the Bering Strait region or vice versa have occurred several times when the Earth was much warmer than now (i.e., hypsithermal times, such as 6000–7000 BP, 120 000 BP, or 15–17 Ma BP). However, the story of the global spread of Trochammina hadai is a typical story of anthropogenic dispersion. Cultivated oysters and oyster larvae from Japan, with their associated packing of plant matter and sediment, were a possible early vector for the introduction of T. hadai into the USA, France, Sweden, and Brazil. These specimens were likely to have been further transported to a secondary location by means of mud on anchors or anchor chains of fishing and pleasure boats. Whereas larger current systems are unlikely to transport viable specimens of T. hadai (adults and propagules) globally, the rise of commercial shipping after World War II exponentially increased the quantity of ballast water and sediment shipped worldwide, both of which were dumped into receiving ports with no concern about NIS. Despite new efforts to curb these NIS introductions, we now have an unprecedented number of anthropogenic introductions in the last 200 years that, when combined, represent planetary-scale changes to the biosphere and act as potential stratigraphic markers for the Anthropocene (Williams et al., 2022). Where T. hadai is present as an alien species, more studies could help enhance our understanding of the timing of introductions on local and global scales. Whether this occurs by obtaining dateable cores or new techniques such as single-cell transcriptomics, which help connect or separate populations and clarify introduction gateways by studying primary and secondary introduction dynamics (Weiner et al., 2023), it is critical that we address the impact of these microscopic invaders on biodiversity, native populations, and ecosystem functioning through further investigations.
Some data discussed in this paper are available in three U.S. Geological Survey data releases (https://doi.org/10.5066/P13UOJDV, McGann, 2024; https://doi.org/10.5066/P13KSOAC, McGann et al., 2024; https://doi.org/10.5066/P9QQT6KZ, McGann and Lorenson, 2025). At the time of publication, (1) unpublished census counts of foraminifera in ballast sediment samples of the Great Lakes of the USA were not available from the University of Michigan and the National Oceanic and Atmospheric Administration; (2) presence or absence data of Trochammina hadai in San Francisco Bay were not available from Stanford University; (3) foraminiferal census counts from San Francisco Bay were not available from the University of California, Berkeley; and (4) foraminiferal census counts from Sweden were not available from the University of Gothenburg. Repository information regarding the figured specimens of T. hadai is presented in Table S2 in the Supplement.
The supplement related to this article is available online at https://doi.org/10.5194/jm-44-275-2025-supplement.
MM performed the sampling, picking, and identification of living T. hadai individuals along the western US seaboard and constructed the time series for San Francisco Bay. HK and MT (Japan); VMPB and JCP (France); IPA, JCP, PO, JA, and MA (Sweden); CMT and DWH (Australia); SH and MM (USA); and STD, PPBE, and ARR (Brazil) reported on global occurrences of T. hadai. MH and MT provided the molecular data and SEM images. MH, JCP, PPBE, IPA, ARR, CMT, DWH, STD, and MM provided the light microscope images. MW contributed the discussion on the utility of using T. hadai to identify recently accumulating “Anthropocene” strata, DWH with the paleontological record of Trochamminids, and MM with the vectors, pathways, and spreading rates responsible for the prolific global dispersal of T. hadai. All authors contributed to the writing of this article.
The contact author has declared that none of the authors has any competing interests.
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This article is part of the special issue “Advances and challenges in modern and benthic foraminifera research: a special issue dedicated to Professor John Murray”. It is not associated with a conference.
We would like to thank all our present and past colleagues who supplied us with literature and samples documenting distributional pattern of benthic foraminifera along the west coast of the USA from 1930–2016, many of which were associated with the McGann et al. (2024) USGS data release. Thanks go to the crew of the oceanographic vessel Sepia II, Louis Lanoy, Jean-Claude Dauvin, Pierre Balay, and Jean-Philippe Pezy, for their help during sampling in Le Havre, and the crew of the USGS PCMSC R/V Jewell, Daniel Powers and Peter Dal Ferro, for collecting the 2024 San Francisco Bay cores. Rowan Azhderian and Kevin Arizmendi of the USGS PCMSC Core Preparation and Analysis Laboratory under the direction of core lab manager Jason Padgett enthusiastically photographed, X-Ray/CT-scanned, split, and sampled the cores, as well as preparing the radiochemistry samples. Brian Huber of the National Museum of Natural History, Washington, D.C., USA, kindly scheduled and provided Sibelle Trevisan Disaró access to their museum facilities, the Cushman Foraminiferal Collection, and their FossilLab with an EDS stereomicroscope and photographic equipment. Ruth Martin kindly provided us access to the Washington State Department of Ecology samples that are now housed at the Burke Museum at the University of Washington, USA, and Amy Klecker, Michael Weaver, and Martin Trinh of the San Francisco Estuary Institute provided access to archived Regional Monitoring Program samples from 2014–2016. Andrew Cohen shared excellent comments on an earlier draft of the manuscript. Special thanks go to our editor, Andy Gooday, as well as Bruce Hayward, Martin Langer, and Anna Weinmann, for their extremely helpful reviews. We would particularly like to acknowledge the contributions of Doris Sloan, University of California, Berkeley, for her decades-long substantive contributions to the Trochammina hadai invasion story. This article is a contribution to a JM special issue dedicated to John W. Murray, whose books, articles, and presence have influenced and encouraged foraminiferal workers globally through the ages.
This research was funded in part by the Transport of Invasive Microorganisms task of the Sediment Transport in Coastal Environments project of the USGS Pacific Coastal and Marine Science Center (Mary McGann). The molecular work was supported by a Schmidheiny Foundation grant (SHF-2023) (Maria Holzmann). Records in Normandy (France) were part of the Foram-INDIC project and received financial support from the “Agence de l'Eau Artois Picardie” and the “Agence de l'Eau Seine Normandie” (grant nos. 58183 and 1082222, 2019, respectively) (Vincent M. P. Bouchet and Jean-Charles Pavard). Data from Långedrag and Fiskebäckskil, Sweden, come from the FORALIEN project, which has received funding from the European Union's Horizon 2023 research and innovation program under a Marie Skłodowska-Curie grant (grant agreement no. 101151826; Jean-Charles Pavard and Irina Polovodova).
This paper was edited by Andy Gooday and reviewed by Bruce Hayward, Martin R. Langer, and Anna E. Weinmann.
Akaboshi, S.: Notas sobre o comportamento da ostra japonesa, Crassostrea gigas (Thunberg, 1793) no litoral do Estado de São Paulo, Brasil, Bol. Inst. Pesca, 6, 93–104, 1979.
Akaboshi, S., Pereira O. M., and Singue, C.: Cultivo experimental de Crassostrea gigas (Thunberg, 1793), na região estuarina lagunar de Cananéia (25°050S; 48°010W), São Paulo Brasil, Bol. Inst. Pesca, 10, 1–8, 1983.
Alexander, C. R. and Lee, H. J.: Sediment accumulation on the Southern California Bight continental margin during the twentieth century, in: Earth Science in the Urban Ocean: The Southern California Continental Borderland, edited by: Lee, H. J., and Normark, W. R., Special Paper 454, Geological Society of America, Boulder, CO, USA, 69–87, https://doi.org/10.1130/2009.2454(2.4), 2009.
Alexander, C. R. and Venherm, C.: Modern sedimentary processes in the Santa Monica, California continental margin: Sediment accumulation, mixing and budget, Mar. Environ. Res., 56, 177–204, 2003.
Alve, E.: Opportunistic features of the foraminifer Stainforthia fusiformis (Williamson): evidence from Frierfjord, Norway, J. Micropalaeontol., 13, 24–24, https://doi.org/10.1144/jm.13.1.24, 1994.
Alve, E. and Goldstein, S. T.: Resting stage in benthic foraminiferal propagules: a key feature for dispersal? Evidence from two shallow-water species, J. Micropalaeontol., 21, 95–96, https://doi.org/10.1144/jm.21.1.95, 2002.
Alve, E. and Goldstein, S. T.: Propagule transport as a key method of dispersal in benthic foraminifera. Limnol. Oceanogr., 48, 2163–2170, 2003.
Alve, E. and Goldstein, S. T.: Dispersal, survival and delayed growth of benthic foraminiferal propagules, J. Sea Res., 63, 36–51, 2010.
Andrews, J.: A review of introductions of exotic oysters and biological planning for new importations, Mar. Fish. Rev., 42, 1–11, 1980.
Arcachon Bay: The Origins of Oyster Farming: https://huitres-arcachon-capferret.fr/en/one-story-one-origin/ (last access: 20 May 2025), 2024.
Asplund, J.: Current and historical ecological quality status of Skärhamn, Bachelor Thesis, University of Gothenburg, Sweden, 37 pp., 2024.
Axelsson, M.: Foraminiferer som indikatorer av miljöstatus i Hakefjorden/Foraminifera as indicators of environmental quality status in the Hake Fjord, Bachelor thesis, University of Gothenburg, Sweden, 41 pp., 2024 (in Swedish).
Bailey, S. A., Duggan, I. C., Jenkins, P. T., and Macisaac, H. J.: Invertebrate resting stages in residual ballast sediment of transoceanic ships, Can. J. Fish. Aquat. Sci., 62, 1090–1103, 2005.
Baskaran, M.: Dating of biogenic and inorganic carbonates using 210Pb-226Ra disequilibrium method: A review, in: Handbook of Environmental Isotope Geochemistry, edited by: Baskaran, M., Springer, Berlin/Heidelberg, 789–809, 2011.
Bertelsmeier, C., Ollier, S., Liebhold, A. M., Brockerhoff, E. G., Ward, D., and Keller, L.: Recurrent bridgehead effects accelerate global alien ant spread, P. Natl. Acad. Sci.-Biol., 115, 5486–5491, https://doi.org/10.1073/pnas.1801990115, 2018.
Bird, C., Schweizer, M., Roberts, A., Austin, W. E. N., Knudsen, K. L., Evans, K. M., Filipsson, H. L., Sayer, M. D. J., Geslin, E., and Darling, K. F.: The genetic diversity, morphology, biogeography, and taxonomic designations of Ammonia (Foraminifera) in the Northeast Atlantic, Mar. Micropaleontol., 155, 101726, https://doi.org/10.1016/j.marmicro.2019.02.001, 2020.
Bouchet, V. M. P., Debenay, J. P., and Sauriau, P. G.: First report of Quinqueloculina carinatastriata (Wiesner, 1923) (foraminifera) along the French Atlantic coast (Marennes-Oleron Bay and Ile De Re), J. Foramin. Res., 37, 204–212, https://doi.10.2113/gsjfr.37.3.204, 2007.
Bouchet, V. M. P., Pavard, J.-C., Holzmann, M., McGann, M., Armynot du Châtelet, E., Courleux, A., Pezy, J.-P., Dauvin, J.-C., and Seuront, L.: The invasive Asian benthic foraminifera Trochammina hadai Uchio, 1962: identification of a new local in Normandy (France) and a discussion on its putative introduction pathways, Aquat. Invasions, 18, 23–38, https://doi.org/10.3391/ai.2023.18.1.103512, 2023.
Bremer, G. M. A., Smelror, M., Nagy, J., and Vigran, J. O.: Biotic responses to the Mjølnir meteorite impact, Barents Sea: Evidence from a core drilled within the crater, in: Cratering in marine environments and on ice, edited by: Dypvik, H., Burchell, M. J., and Heidelberg, C. P., Springer-Verlag, 21–31, ISBN 3540406689, 2003.
Bresler, V. and Yanko, V.: Chemical ecology: a new approach to the study of living benthic epiphytic foraminifera, J. Foramin. Res., 25, 267–279, 1995.
Brinkmann, I., Schweizer, M., Singer, D., Quinchard, S., Barras, C., Bernhard, J. M., and Filipsson, H. L.: Through the e DNA looking glass: Responses of fjord benthic foraminiferal communities to contrasting environmental conditions, J. Eurkaryot. Microbiol., 70, e12975, https://doi.org/10.1111/jeu.12975, 2023.
Burone, L. and Pires-Vanin, A. M. S.: Foraminiferal assemblages in the Ubatuba Bay, southeastern Brazilian Coast. Scientia Marina, in: The introduced Northern Pacific seastar Asterias amurensis in Tasmania, edited by: Buttermore, R. E., Turner, E., and Merrill, M. G., Mem. Queensland Mus., 36, 1–25, https://doi.org/10.3989/scimar.2006.70n2203, 1994.
Bush, J. B.: Foraminifera of Tomales Bay, California, Micropaleontol. Bull., 2, 38–42, 1930.
Buttermore, R. E., Turner, E., and Merrill, M. G.: The introduced Northern Pacific seastar Asterias amurensis in Tasmania, Mem. Queensland Mus., 36, 1–25, 1994.
California Association of Port Authorities: Port of San Diego, https://californiaports.org/ports/port-of-san-diego/ (last access: 20 May 2025), 2024.
Canada, Minister of Justice: Ballast Water Control and Management Regulations, SOR/2021-120, https://laws-lois.justice.gc.ca/PDF/SOR-2011-237.pdf (last access: 20 May 2025), 2022.
Calvo-Marcilese, L. and Langer, M. R.: Breaching biogeographic barriers: The invasion of Haynesina germanica (Foraminifera, Protista) in the Bahia Blanca Estuary, Argentina, Bio. Invasions, 12, 3299–3306, 2010.
Carlton, J. T.: Introduced invertebrates of San Francisco Bay, in: San Francisco Bay – the Urbanized Estuary, edited by: Conomos, T. J., American Association for the Advancement of Science, Pacific Division, San Francisco, 427–444, ISBN 0-934394-01-6, 1979.
Carlton, J. T.: Transoceanic and interoceanic dispersal of coastal marine organisms: The biology of ballast water, Oceanogr. Mar. Biol. Ann. Rev., 23, 313–371, 1985.
Carlton, J. T., Chapman, J. W. C., Geller, J. B., Miller, J. A., Carlton, D. A., McCuller, M. I., Treneman, N., Steves, B. P., and Ruiz, G. M.: Tsunami-driven rafting: Transoceanic species dispersal and implications for marine biogeography, Science, 357, 1402–1406, https://doi.org/10.1126/science.aao1498, 2017.
Carlton, J. T. and Geller, J. B.: Ecological roulette: The global transport of non-indigenous marine organisms, Science, 261, 8–82, 1993.
Carlton, J. T. and Hodder, J.: Biogeography and dispersal of coastal marine organisms: experimental studies on a replica of a 16th century sailing vessel, Mar. Biol., 121, 721–730, 1995.
Carlton, J. T., Reid, D. M., and van Leeuwen, H.: Shipping study. The role of shipping in the introduction of non-indigenous aquatic organisms to the coastal waters of the United States (other than the Great Lakes) and analysis of control options, The National Sea Grant College Program/Connecticut Sea Grant Project R/ES-6. Department of Transportation, United States Coast Guard, Washington, D.C. and Groton, Connecticut, Report CG-D-11-95, Government Accession Number AD-A294809, 213 pp., and Appendices A-I (122 pp.), 1995.
Carlton, J. T., Thompson, J. K., Schemel, L. E., and Nichols, F. H.: Remarkable invasion of San Francisco Bay (California, USA) by the Asian clam Potamocorbula amurensis. I. Introduction and dispersal, Mar. Ecol. Prog. Ser., 66, 81–94, 1990.
Casas-Monroy, O., Linley, D. R., Adams, J. K., Chan, F., Drake, D. A. R., and Bailey, S. A.: Relative invasion risk for phytoplankton across marine and freshwater systems: Examining efficacy of proposed international ballast water discharge standards, PLoS One, 10, e0118267, https://doi.org/10.1371/journal.pone.0118267, 2015.
Caswell, D.: Oystering on Willapa Bay Washington oyster farmers are inheritors of a tradition and way of life that goes back to 1851, Our Coast Magazine, https://www.discoverourcoast.com/coast-weekend/coastal-life/oystering-on-willapa-bay-washington-oyster-farmers-are-inheritors-of-a-tradition-and-way-of/article_04e10e47-0f6c-5f0c-b833-86d6b4f0a737.html (last access: 20 May 2025), 2016.
Cearreta, A.: Population dynamics of benthic foraminifera in the Santona Estuary, Spain, Rev. Paléobiol., 2, 721–724, 1988.
Choi, J. U. and An, S.: High benthic foraminiferal diversity in polluted Busan North Port (Korea), J. Foramin. Res., 2, 327–339, 2012.
Chu, K. H., Tam, P. F., Fung, C. H., and Chen, Q. C.: A biological survey of ballast water in container ships entering Hong Kong, Hydrobiologia, 352, 201–206, 1997.
City of Tacoma: Port of Tacoma, https://www.cityoftacoma.org/government/city_departments/community_and_economic_development/economic_development_services/port_of_tacoma#:~:text=The top five trading partners,Alaska%2C South Korea and Taiwan (last access: 20 May 2025), 2024.
Clarke, K. R. and Gorley, R. N.: PRIMER v. 6, User Manual/Tutorial. Primer-E, Plymouth, UK, 190 pp., 2006.
Clémence, M. E., Gardin, S., Bartolini, A., Paris, G., Beaumont, V., and Guex, J.: Bentho-planktonic evidence from the Austrian Alps for a decline in sea-surface carbonate production at the end of the Triassic, Swiss. J. Geosci., 103, 293–315, 2010.
Cohen, A. N. and Carlton, J. T.: Non-indigenous aquatic species in a United States estuary: A case study of the biological invasion of San Francisco Bay and Delta. Report to U.S. Fish and Wildlife Service, Washington, D.C., and National Sea Grant College Program, Connecticut Sea Grant, 246 pp., http://bioinvasions.org/wp-content/uploads/1995-SFBay-Invasion-Report.pdf (last access: 20 May 2025), 1995.
Cohen, A. N. and Carlton, J. T.: Accelerating invasion rate in a highly invaded estuary, Science, 279, 555–558, 1998.
Cohen, A. N., Weinstein, A., Emmett, M. A., Lau, W., and Carlton, J. T.: Investigations into the introduction of non-indigenous marine organisms via the cross-continental trade in marine baitworms, SFEI Contribution No. 357. San Francisco Estuary Institute, Richmond, CA, https://www.sfei.org/documents/investigations-introduction-non-indigenous-marine-organisms-cross-continental-trade (last access: 28 August 2025), 2001.
Cohen, K. M., Finney, S. C., Gibbard, P. L., and Fan, J.-X.: The ICS International Chronostratigraphic Chart, Episodes, 36, 199–204 (updated v2023/04), 2013.
Connor, C. L.: Holocene sedimentation history of Richardson Bay, California, Masters Thesis, Stanford University, Stanford, California, U.S., 112 pp., 1975.
Conomos, T. J.: Geologic aspects of the recent sediments of south San Francisco Bay, Masters Thesis, San Jose State College, San Jose, California, U.S., 118 pp., 1963.
David, M., Gollasch, S., Leppäkoski, E., and Hewitt, C.: Risk Assessment in Ballast Water Management, in: Global Maritime Transport and Ballast Water Management, edited by: David, M., and Gollasch, S., New York, NY, Springer Science & Business Media, 133–169, https://doi.org/10.1007/978-3-031-48193-2, 2015.
Deldicq, N., Alve, E., Schweizer, M., Polovodova Asteman, I., Hess, S., Darling, K., and Bouchet, V. M. P.: History of the introduction of a species resembling the benthic foraminifera Nonionella stella in the Oslofjord (Norway): Morphological, molecular and paleo-ecological evidences, Aquat. Invasions, 14, 182–205, https://doi.10.3391/ai.2019.14.2.03, 2019.
Denton, C., Bussiéres, E., Jiang, R., and Thompson, R.: The physics of foraminifera. Bioengineering Hyperbook, Department of Bioengineering, McGill University, https://bioengineering.hyperbook.mcgill.ca/the-physics-of-foraminifera/ (last access: 20 May 2025), 2025.
de Rivera, C. E., Steves, B. P., Ruiz, G. M., Fofonoff, P., and Hines, A. H.: Northward spread of marine non-indigenous species along western North America: forecasting risk of colonization in Alaskan waters using environmental niche modeling, Prince William Sound Regional Citizens' Advisory Council and U.S. Fish and Wildlife Service, 36 pp., 2006.
Diederich, S., Nehls, G. J. E. E., Van Beusekom, J. E., and Reise, K.: Introduced Pacific oysters (Crassostrea gigas) in the northern Wadden Sea: invasion accelerated by warm summers?, Helgoland Mar. Res., 59, 97–106, 2005.
Dinnel, P. A., Peabody, B., and Peter-Contesse, T.: Rebuilding Olympia oysters, Ostrea lurida Carpenter 1864, in Fidalgo Bay, Washington, J. Shellfish Res., 28, 79–85, 2009.
Dobbs, F. C., Diallo, A. A., Doblin, M. A., Drake, L. A., Graczyk, T., Jiang, X., Johengen, T. H., Kampschmidt, L., Rublee, P., Reid, D. F., and Ruiz, G.: Pathogens in ships' ballast water and sediment residuals. Third International Conference on Marine Bioinvasions, San Diego, CA, https://caseagrant.ucsd.edu/sites/default/files/importedFiles/mbi3_abstract_book.pdf (last access: 28 August 2025), 2023.
Drake, L., Choi, K., Ruiz, G., and Dobbs, F.: Global redistribution of bacterioplankton and virioplankton communities, Bio. Invasions, 3, 193–199, 2001.
Drake, L. A., Jenkins, P. T., and Dobbs, F. C.: Domestic and international arrivals of NOBOB (no ballast on board) vessels to lower Chesapeake Bay, Mar. Pollut. Bull., 50, 560–565, 2005.
Drexler, J. Z., Fuller, C. C., and Archfield, S.: The approaching obsolescence of 137 Cs dating of wetland soils in North America, Quaternary Sci. Rev., 199, 83–96, 2018.
Drinkwaard, A.: Introductions and developments of oyster in the North Sea area: a review, Helgolander Meeresun., 52, 301–308, 1999.
Duggan, I. C., Bailey, S. A., Van Overdijk, C. D. A., and Macisaac, H. J.: Invasion risk of active and diapausing invertebrates from residual ballast in ships entering Chesapeake Bay, Mar. Ecol. Prog. Ser., 324, 57–66, 2006.
Duggan, I. C., Van Overdijk, C. D. A., Bailey, S. A., Jenkins, P. T., Limen, H., and Macisaac, H. J.: Invertebrates associated with residual ballast water and sediments of cargo-carrying ships entering the Great Lakes, Can. J. Fish. Aquat. Sci., 62, 2463–2474, 2005.
Duleba, W.: Interpretações paleoambientais obtidas a partir dasvariações na coloração das carapaças de foraminíferos, da Enseadado Flamengo, SP. Bol. Inst. Oceanogra., 42, 63–72, https://doi.org/10.1590/S0373-55241994000100005, 1994.
Duleba, W., Debenay, J. P., Eichler, B. B., and Mahiques, M. M.: Holocene environmental and water circulation changes: Foraminifer morphogroups evidence, Flamengo Bay (SP, Brazil), J. Coastal Res., 15, 554–571, 1999.
Eichler, P. P. B.: Evaluation of seasonal anoxia in Florianópolis North Bay through environmental indicators, J. Aquat. Mar. Biol., 13, 31–35, https://doi.org/10.15406/jamb.2024.13.00393, 2024.
Eichler, P. P. B., McGann, M., Rodrigues, A. R., Mendonça, A., Amorim, A., Bonetti, C., Cordeiro de Farias, C., Mello e Sousa, S. H., Vital, H., and Praxedes Gomes, M.: The occurrence of the invasive foraminifera Trochammina hadai Uchio in Flamengo Inlet, Ubatuba, São Paulo State, Brazil, Micropaleontol., 64, 391–402, 2018.
Emami, K., Askari, V., Ullrich, M., Mohinudeen, K., Anil, A. C., Khandeparker, L., Burgess, J. G., and Mesbahi, E.: Characterization of bacteria in ballast water using MALDI-TOF mass spectrometry, PLoS One, 7, e38515, https://doi.org/10.1371/journal.pone.0038515, 2012.
Ertan, K. T., Hemleben, V., and Hemleben, C.: Molecular evolution of some selected benthic foraminifera as inferred from sequences of the small subunit ribosomal DNA, Mar. Micropaleontol., 53, 367–388, 2004.
Esveldt, G. D.: A review of the oyster industry of the State of Washington, https://digitalarchives.wa.gov/do/9F62B519F9A253BC3D7EFA42048FDE65.pdf (last access: 20 May 2025), 1948.
Faria, L., Frehse, F. D. A., Occhi, T. V. T., Carvalho, B. M. D., Pupo, D. V., Disaró, S. T., and Vitule, J. R. S.: Occurrence of non-native species in a subtropical coastal River, in Southern Brazil, Acta Limnologica Brasiliensia, 33, e101, https://doi.org/10.1590/S2179-975X2320, 2021.
Fernandes, L., Zehnder-Alves, L., and Bassfeld, J.: The recently established diatom Coscinodiscus wailesii (Coscinodiscales, Bacillariophyta) in Brazilian waters. I: Remarks on morphology and distribution, Phycol. Res., 49, 89–96, https://doi.org/10.1046/j.1440-1835.2001.00226.x, 2001.
Finger, K. L.: Tsunami-generated rafting of foraminifera across the North Pacific Ocean, Aquat. Invasions, 13, 17–30, https://doi.org/10.3391/ai.2018.13.1.03, 2018.
Fregoso, T. A., Foxgrover, A. C., and Jaffe, B. E.: Sediment deposition, erosion, and bathymetric change in central San Francisco Bay: 1855–1979, United States Geological Survey Open-File Report 2008-1312, 41 pp., https://doi.org/10.3133/ofr20081312, 2008.
Fuller, C. C., van Geen, A., Baskaran, M., and Anima, R.: Sediment chronology in San Francisco Bay, California, defined by 210Pb, 234Th, 137Cs, and 239, 240Pu, Mar. Chem., 64, 7–27, https://doi.org/10.1016/S0304-4203(98)00081-4, 1999.
Galil, B. S. and Hülsmann, N.: Protist transport via ballast water – biological classification of ballast tanks by food web interactions, Eur. J. Protistol., 33, 244–253, 1997.
Gizel, H. and Héral, M.: Introduction into France of the Japanese oyster (Crassostrea gigas), J. Cons. Int. Explor. Mer., 47, 399–403, 1991.
Goldbeck, E., Houben, C., and Langer, M. R.: Survival of foraminifera in the gut of holothuroids from Elba Island Mediterranean Sea, Rev. de Micropaleontol., 48, 66–174, 2005.
Golden Gate Weather Services: Climate of San Francisco, Sorted Seasonal Rainfall, https://ggweather.com/sf/season.html (last access: 20 May 2025), 2024.
Golebiowski, R.: Facial and faunistic changes from Triassic to Jurassic in the northern Calcareous Alps, Cah. de l'Université de Lyon, 3, 175–184, 1990.
Gollasch, S.: The importance of ship hull fouling as a vector of species introductions into the North Sea, J. Bioadhesion Biofilm Res., 18, 105–121, https://doi.org/10.1080/08927010290011361, 2010.
Gollasch, S., Dammer, M., Lenz, J., and Andres, H. G.: Non-indigenous organisms introduced via ships into German waters, in: Ballast Water: Ecological and Fisheries Implications, edited by: Carlton, J. T., ICES Conference (83rd Statutory Meeting), 21–29 September 1995, Aalborg, Denmark. Cooperative Research Report No. 224, International Council for the Exploration of the Sea, Copenhagen, 50–64, https://doi.org/10.17895/ices.pub.7681, 1998.
Gollasch, S., Lenz, J., Dammer, M., and Andres, H. G.: Survival of tropical ballast water organisms during a cruise from the Indian Ocean to the North Sea, J. Plankton Res., 22, 923–937, 2000.
Gollasch, S., Macdonald, E., Belson, S., Botnen, H., Christensen, J. T., Hamer, J. P., Houvenaghel, G., Jelmert, A., Lucas, I., Masson, D., McCollin, T., Olenin, S., Persson, A., Wallentinus, I., Wetsteyn, L. P. M. J., and Wittling, T.: Life in ballast tanks, in: Invasive Species of Europe-Distribution, Impact and Management, edited by: Leppäkoski, E., Gollasch, S., and Olenin, S., Dordrecht, Kluwer, 217–231, https://doi.org/10.1007/978-94-015-9956-6_23, 2002.
Gooday, A. J.: Deep-sea benthic foraminiferal species which exploit phytodetritus: Characteristic features and controls on distnbution, Mar. Micropaleontol., 22, 187–205, 1993.
Gouy, M., Guindon, S., and Gascuel, O.: SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building, Mol. Biol. Evol., 27, 221–224, 2010.
Gram, R.: The marine geology of the Recent sediments of central San Francisco Bay, Masters Thesis, San Jose State College, San Jose, California, U.S., 132 pp., 1966.
Granmo, M. N., Reavie, E. D., TenEyck, M. C., Branstrator, D. K., Schwerdt, T., Cangelosi, A. A., and Cai, M.: Evaluation of a method for ballast water risk–release assessment using a protist surrogate, Hydrobiologia, 817, 11–22, https://doi.org/10.1007/s10750-018-3517-z, 2018.
Greeve, Y.: The Pacific oyster has rapidly taken over in the shallow bays of Bohuslän on Sweden's west coast, The Faculty of Science, University of Gothenburg, https://www.gu.se/en/news/asian-oysters-are-dominant-on-swedens-west-coast (last access: 20 May 2025), 2023.
Gross, O.: Influence of temperature, oxygen and food availability on the migrational activity of bathyal benthic foraminifera: Evidence by microcosm experiments, Hydrobiologia, 426, 123–137, 2000.
Guastella, R., Marchini, A., Caruso, A., Evans, J., Cobianchi, M., Cosentino, C., Langone, L., Lecci, R., and Mancin, N.: Reconstructing bioinvasion dynamics through micropaleontologic analysis highlights the role of temperature change as a driver of alien foraminifera invasion, Front. Mar. Sci., 8, 675807, https://doi.org/10.3389/fmars.2021.675807, 2021.
Guindon, S., Dufayard, J. F., Lefort, V., Anisimova, M., Hordijk, W., and Gascuel, O.: New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0, Syst. Biol., 59, 307–321, 2010.
Guy-Haim, T., Hyams-Kaphzan, O., Yeruham, E., Almogi-Labin, A., and Carlton, J. T.: A novel marine bioinvasion vector: ichthyochory, live passage through fish, Limnol. Oceanogr. Lett., 2, 81–90, https://doi.10.1002/lol2.10039, 2017.
Harrington, G. L.: Ammobaculites, migrant or recent introduction to California?, Contribution to the Cushman Foundation for Foraminiferal Research, 7, 9–30, 1956.
Hayek, L. A. C. and Buzas, M. A.: Surveying natural populations. Columbia University Press, New York, 563 pp., ISBN 9780231146203, 1997.
Hayward, B. W.: Introduced marine organisms in New Zealand and their impact in the Waitemata Harbour, Auckland. Tane, 36, 197–223, 1997.
Hayward, B. W., Figueira, B. O., Sabaa, A. T., and Buzas M. A.: Multi-year life spans of high salt marsh agglutinated foraminifera from New Zealand, Mar. Micropaleontol., 109, 54–65, 2014.
Hayward, B. W., Grenfell, H. R., Reid, C. M., and Hayward, K. A.: Recent New Zealand shallow-water benthic foraminifera: Taxonomy, ecologic distribution, biogeography, and use in paleoenvironmental assessment, Institute of Geological and Nuclear Science Monograph 21, New Zealand Geological Survey Paleontological Bulletin, 75, 258 pp., ISBN 0478096623, ISBN 9780478096620, 1999.
Hayward, B. W., Holzmann, M., Grenfell, H. R., Pawlowski, J., and Triggs, C. M.: Morphological distinction of molecular types in Ammonia towards a taxonomic revision of the world's most commonly misidentified foraminifera, Mar. Micropaleontol., 50, 237–271, 2004.
Hayward, B. W., Holzmann, M., Pawlowski, J., Parker, J. H., Kaushik, T., Toyofuku, M. S., and Masashi, T.: Molecular and morphological taxonomy of living Ammonia and related taxa (Foraminifera) and their biogeography, Micropaleontology, 67, 109–313, 2021.
Headlee, L.: Transportation of Foraminifera by Ducks, University of Southern California, term paper, 1961.
Hewitt, C., Campbell, M., Thresher, R., Martin, R., Boyd, S., Cohen, B., Currie, D., Gomon, M., Keough, M., Lewis, J., Lockett, M., Mays, N., McArthur, M., O'Hara, T., Poore, G., Ross, J., Storey, M., Watson, J., and Wilson, R.: Introduced and cryptogenic species in Port Phillip Bay, Victoria, Australia, Mar. Biol., 144, 183–202, 2004.
Hickman, C. S. and Lipps, J. H.: Foraminiferivory: Selective ingestion of foraminifera and test alterations produced by the neogastropod Olivella, J. Foram. Res., 13, 108–114, 1983.
Himson, S., Williams, M., Zalasiewicz, J., Waters, C., McGann, M., England, R., Jaffe, B. E., Boom, A., Holmes, R., Sampson, S., Pye, C., Berrio, J. C., Tyrrell, G., Wilkinson, I. P., Rose, N., Gaca, P., and Cundy, A.: The San Francisco Estuary, USA as a reference section for the Anthropocene series, Anthropocene Rev., 10, 87–115, 2023.
Holzmann, M.: Isolation, DNA extraction, amplification and gel electrophoresis of single-celled nonmarine foraminifera (Rhizaria), in: Practical Handbook on Soil Protists, edited by: Amaresan, N. and Chandarana, K. A., 181–188, https://doi.org/10.1007/978-1-0716-3750-0, 2024.
Hülsmann, N. and Galil, B. S.: Protists – A dominant component of the ballast-transported biota, in: Invasive Aquatic Species of Europe. Distribution, Impacts and Management, edited by: Leppäkoski, E., Gollasch, S., and Olenin, S., Dordrecht, Springer, 20-26, https://doi.org/10.1007/978-94-015-9956-6_3, 2002.
Hurlbert, S. H.: The nonconcept of species diversity: a critique and alternative parameters, Ecology, 52, 577–586, 1971.
Hyams, O., Almogi-Labin, A., and Benjamini, C.: Larger foraminifera of the southeastern Mediterranean shallow continental shelf off Israel, Israel J. Earth Sci., 51, 169–179, 2002.
Ikeya, N.: Ecology of foraminifer in the Haman Lake region on the Pacific Coast of Japan, Reports of the Faculty of Science, Shizuoka University, 11, 131–159, https://gbank.gsj.jp/ld/resource/geolis/197700430 (last access: 20 May 2025), 1977.
International Maritime Organization: Regulation D-2 Ballast Water Performance Standard, https://www.imo.org/en/about/conventions/pages/international-convention-for-the-control-and-management-of-ships'-ballast-water-and-sediments-(bwm).aspx (last access: 20 May 2025), 2004.
International Maritime Organization: Antifouling systems, https://www.imo.org/en/ourwork/environment/pages/anti-fouling.aspx (last access 20 May 2025), 2024.
Jauffrais, T., Jesus, B., Geslin, E., Briand, F., and Martin Jézéquel, V.: Locomotion speed of the benthic foraminifer Ammonia tepida exposed to different nitrogen and carbon sources, J. Sea Res., 118, 52–58, 2016.
Johannessen, S. C. and Macdonald, R. W.: There is no 1954 in that core! Interpreting sedimentation rates and contaminant trends in marine sediment cores, Mar. Pollut. Bull., 64, 675–678, 2012.
Johengen, T., Reid, D., Fahnenstiel, G., MacIsaac, H., Dobbs, F., Doblin, M., and Jenkins, P.: Assessment of transoceanic NOBOB vessels and low-salinity ballast water as vectors for non-indigenous species introductions to the Great Lakes. Cooperative Institute for Limnology and Ecosystems Research, School of Natural Resources and Environment, University of Michigan, http://glpf.org/wp/wpcontent/uploads/2011/03/nobob_a_final_report.pdf (last access: 20 May 2025), 2005.
Jokiel, P. L.: Long distance dispersal of reef corals by rafting, Coral Reefs, 3, 113–116, 1984.
Jorissen, F. J.: Colonization by the benthic foraminifer Rosalina (Tretomphalus) concinna of Mediterranean drifting plastics. The Mediterranean Science Commission Workshop (CIESM) Monographs No. 46, Marine litter in the Mediterranean and Black Seas, 87–95, https://ciesm.org/online/monographs/46/CIESM_Monograph_46_Marine_Plastic_Litter_87_95.pdf (last access: 20 May 2025), 2014.
Katsanevakis, S., Zenetos, A., Corsini-Foka, M., and Tsiamis, K.: Biological Invasions in the Aegean Sea: Temporal Trends, Pathways, and Impacts, in: The Handbook of Environmental Chemistry, Berlin, Heidelberg, Springer, https://doi.org/10.1007/698_2020_642, 2020.
Kim, S., Frontalini, F., Martins, V., and Lee, W.: Modern benthic foraminiferal diversity of Jeju Island and initial insights into the total foraminiferal diversity of Korea, Mar. Biodiv., 46, 337–354, https://doi.org/10.1007/s12526-015-0364-2, 2016.
Kitazato, H. and Matsushita, S.: Laboratory observations of sexual and asexual reproduction of Trochammina hadai Uchio, Transactions and Proceedings of the Palaeontological Society of Japan, New Series, 182, 454–466, 1996.
Kornicker, L. S.: Spread of ostracodes to exotic environs on transplanted oysters, B. Am. Paleontol., 65, 129–139, 1975.
Koutsoukos, E. A. M. and Hart, M. B.: Cretaceous foraminiferal morphogroup distribution patterns, paleocommunities and trophic structures: a case-study from the Serpige Basin, Brazil, T. Roy. Soc. Edin.-Earth, 81, 221–246, 1990.
Kuerschner, W. M., Bonis, N. R., and Krystyn, L.: Carbon isotope stratigraphy and palynostratigraphy of the Triassic-Jurassic transition in the Tiefengraben section–Northern Calcareous Alps (Austria), Palaeogeogr. Palaeoclim., 244, 257–280, 2007.
Langer, M. R.: Epiphytic foraminifera, Mar. Micropaleontol., 20, 235–265, 1993.
Langer, M. R. and Hottinger, L., and Huber, B.: Functional morphology in low-diverse benthic foraminiferal assemblages from tidal-flats of the North Sea, Senck. Marit., 20, 81–99, 1989.
Langer, M. R. and Leppig, U.: Molecular phylogenetic status of Ammonia catesbyana (d'Orbigny 1839), an intertidal foraminifer from the North Sea, Neues Jahrb. Geol. P.-A., 9, 545–556, 2000.
Langer, M. R., Weinmann, A. E., Lötters, S., and Rödder, D.: “Strangers” in paradise: Modeling the biogeographic range expansion of the foraminifera Amphistegina in the Mediterranean Sea, J. Foramin. Res., 42, 234–244, 2012.
Lau, W.: Importation of baitworms and shipping seaweed: Vectors for introduced species?, in: Environmental Issues: From a Local to a Global Perspective, edited by: Sloan, D. and Kelso, D., Environmental Sciences Group Major, University of California, Berkeley, 21–38, 1995.
Lavoie, D. M., Smith, L. D., and Ruiz, G. M.: The potential for intracoastal transfer of non-indigenous species in the ballast water of ships, Estuar. Coast. Shelf S., 48, 551–564, 1999.
Lavoie, R. E.: Oyster culture in North America history, present and future. The 1st International Oyster Symposium Proceedings, Oyster Research Institute News No. 17, https://worldoyster.org/wp/wp-content/uploads/2019/04/news_17e.pdf (last access: 20 May 2025), 2019.
Le Borgne, M., Gras, M. P., Comps, M., Carruesco, G., and Razet, D.: Observations sur la reproduction des huîtres dans la Seudre (Bassin de Marennes-Oléron) en 1972, ICES CM 1983/K, 16, 5 pp., 1973.
Lee, J. J., Faber, W. W., Anderson, O. R., and Pawlowski, J.: Life cycles of foraminifera, in: Biology of Foraminifera, edited by: Lee, J. J., Faber, W. W., Anderson, O. R., and Pawlowski, J., Academic Press, 285–334, ISBN 012440670X, ISBN 9780124406704, 1991.
Lee, Y. G., Choi, Y. H., Jeong, D. U., Lee, J. S., Kim, Y. W., Park, J. J., and Choi, J. U.: Effect of abalone farming on seawater movement and benthic foraminiferal assemblage of Zostera marina in the inner bay of Wando, South Korea. Mar. Pollut. Bull., 109, 205–220, https://doi.org/10.1016/j.marpolbul.2016.05.081, 2016a.
Lee, Y. G., Jeong, D. U., Lee, J. S., Choi, Y. H., and Lee, M. O.: Effects of hypoxia caused by mussel farming on benthic foraminifera in semi-closed Gamak Bay, South Korea, Mar. Pollut. Bull., 109, 566–581, https://doi.org/10.1016/j.marpolbul.2016.01.024, 2016b.
Lefort, V., Longueville, J. E., and Gascuel, O.: SMS: Smart Model Selection in PhyML, Mol. Biol. Evol., 34, 2422–2424, 2017.
Lei, Y. and Li, T.: Atlas of Benthic Foraminifera from China Seas. The Bohai Sea and the Yellow Sea, Springer, Beijing, 399 pp., https://doi.org/10.1007/978-3-662-53878-4, 2016.
Lesen, A. E.: Relationship between benthic foraminifera and food resources in South San Francisco Bay, California, USA, Mar. Ecol. Prog. Ser., 297, 131–145, 2005.
Locke, J. L.: Sedimentation and foraminiferal aspects of the recent sediments of San Pablo Bay, Masters Thesis, San Jose State College, San Jose, California, U.S., 100 pp., 1971.
Loeblich Jr., A. R. and Tappan, H.: Foraminiferal Genera and Their Classification. Van Nostrand Reinhold Company, New York, 970 pp., https://doi.org/10.1007/978-1-4899-5760-3, 1988.
Macdonald, E. M.: Dinoflagellate resting cysts and ballast water discharge in Scottish ports, in: Ballast Water: Ecological and Fisheries Implications, edited by: Carlton, J. T., ICES Conference (83rd Statutory Meeting), 21–29 September 1995, Aalborg, Denmark, Cooperative Research Report No. 224, International Council for the Exploration of the Sea, Copenhagen, 24–35, https://doi.org/10.17895/ices.pub.7681, 1998.
Massé, C., Viard, F., Humbert, S., Antajan, E., Auby, I., Bachelet, G., Bernard, G., Bouchet, V. M. P., Burel, T., Dauvin, J.-C., Delegrange, A., Derrien-Courtel, S., Droual, G., Gouillieux, B., Goulletquer, P., Guérin, L., Janson, A.-L., Jourde, J., Labrune, C., Lavesque, N., Leclerc, J. C., Le Duff,. M., Le Garrec, V., Noël, P., Nowaczyk, A., Pergent-Martin, C., Pezy, J.-P., Raoux, A., Raybaud, V., Ruitton, S., Sauriau, P.-G., Spilmont, N., Thibault, D., Vincent, D., and Curd, A.: An overview of marine non-indigenous species found in the three contrasted biogeographic metropolitan French regions: insights on distribution, origins and pathways of introduction, Diversity, 15, 161, https://doi.org/10.3390/d15020161, 2023.
Matoba, Y.: Distribution of recent shallow water foraminifera of Matsushima Bay, Miyagi Prefecture, northeast Japan, Science Reports of the Tohoku University, Sendai, Second Series (Geology), 42, 1–85, https://tohoku.repo.nii.ac.jp/records/12008 (last access: 2024), 1970.
Matsushita, S. and Kitazato, H.: Seasonality in the benthic foraminiferal community and the life history of Trochammina hadai Uchio in Hamana Lake, Japan, in: Paleoecology, Biostratigraphy, Paleoceanography and Taxonomy of Agglutinated Foraminifera, edited by: Hemleben, C., Kuhnt, W., Kaminski, M. A., and Scott, D. B., the Netherlands, Kluwer Academic Publishers, 695–715, CRID 1573668925350012160, 1990.
McCarthy, S. A. and Khambaty, F. M.: Internal dissemination of epidemic Vibrio cholerae by cargo ship ballast and other nonpotable waters, App. Environ. Microbiol., 60, 2597–2601, 1994.
McDonald, J. A. and Diediker, P. L.: A preliminary report on the foraminifera of San Francisco Bay, California, Micropaeontol. Bull., 2, 33–37, 1930.
McGann, M.: 3500-year B. P. record of climatic change in estuarine deposits of south San Francisco Bay, California, in: Recent geologic studies in the San Francisco Bay area, edited by: Sanginés, E. M., Andersen, D. W., and Buising, A. V., Pacific Section, Society of Sedimentary Geology (SEPM) 76, 225–236, 1995.
McGann, M.: High-resolution foraminiferal, isotopic, and trace element records from Holocene estuarine deposits of San Francisco Bay, California, J. Coastal Res., 24, 1092–1109, 2008.
McGann, M.: Earliest record of the invasive foraminifera Trochammina hadai in San Francisco Bay, California, USA, Mar. Biodivers. Rec., 7, e94, https://doi.org/10.1017/S1755267214000888, 2014.
McGann, M.: Initial dispersal (1986–1987) of the invasive foraminifera Trochammina hadai Uchio in San Francisco Bay, California, USA, Micropaleontology, 64, 365–378, 2018.
McGann, M.: Census counts of the non-indigenous benthic foraminifera Trochammina hadai Uchio obtained in 1983–2024 in San Francisco Bay, U.S. Geological Survey, California, U.S. Geological Survey data release (ver. 2.0, April 2025) [data set], https://doi.org/10.5066/P13UOJDV, 2024.
McGann, M. and Holzmann, M.: First occurrence of the non-indigenous Asian foraminifera Ammonia confertitesta in the northeastern Pacific Ocean: Vancouver Island, British Columbia, Canada, Micropaleontol., 70, 115–127, 2024.
McGann, M. and Lorenson, T. D.: Radiochemistry (210Pb and 137Cs) of cores BC01 and BC02 obtained in 2024 off Alameda and Point Isabel in San Francisco Bay, California, U.S. Geological Survey data release [data set], https://doi.org/10.5066/P9QQT6KZ, 2025.
McGann, M., Sloan, D., and Cohen, A. N.: Invasion by a Japanese marine microorganism in western North America, Hydrobiologia, 421, 25–30, 2000.
McGann, M., Johengen, T. H., Reid, D. F., Ruiz, G. M., and Hines, A. H.: Ballast sediment: a likely mechanism for non-indigenous foraminiferal introductions, Abstracts with Programs, 2003 Annual Meeting, Geol. Soc. Am., 35, 503, https://gsa.confex.com/gsa/2003AM/webprogram/Paper67492.html (last access: 20 May 2025), 2003.
McGann, M., Grossman, E. E., Takesue, R. K., Penttila, D., Walsh, J. P., and Corbett, R.: Arrival and expansion of the invasive foraminifera Trochammina hadai Uchio in Padilla Bay, Washington, Northwest Science, 86, 9–26, 2012.
McGann, M., Erikson, L., Wan, E., Powell II, C., and Maddocks, R. F.: Distribution of biologic, anthropogenic, and volcanic constituents as a proxy for sediment transport in the San Francisco Bay Coastal System, Mar. Geol., 345, 113–142, https://doi.org/10.1016/j.margeo.2013.05.006, 2013.
McGann, M., Ruiz, G. M., Hines, A. H., and Smith, G.: A ship's ballasting history as an indicator of foraminiferal invasion potential – an example from Prince William Sound, Alaska, USA. J. Foramin. Res., 49, 434–455, 2019a.
McGann, M., Schmieder, R. W., and Loncke, L.-P.: Shallow-water foraminifera and other microscopic biota of Clipperton Island, tropical eastern Pacific, Atoll Res. Bull., 626, 1–28, https://doi.org/10.5479/si.10329962.v1, 2019b.
McGann, M., Foxgrover, A. C., Takesue, R. K., Grossman, E. E., Torresan, M. E., Moreno, M., Weakland, S., Keaten, R., Lewis, R., Anima, R. J., Fuller, C. C., Quinterno, P. J., Cohen, A. N., Martin, R. A., Hines, A. H., Wright, C. A., Macdonald, V., Best, M. M. R., Llanso, R. J., Brancato, M. S., Penttila, D., Carlton, J. T., Sloan, D., and Brunner, C. A.: Survey of sites for the presence of the non-indigenous benthic foraminifera Trochammina hadai along the west coast of North America from 1930 to 2024. U.S. Geological Survey data release, U.S. Geological Survey (ver. 2.0, April 2025) [data set], https://doi.org/10.5066/P13KSOAC, 2024.
Means, K. D.: Sediments and foraminifera of Richardson Bay, California, Masters Thesis, University of Southern California, Los Angeles, California, U.S., 80 pp., 1965.
Melo, C. M. R., Silva, F. C., Gomes, C. H. A. M., Solé-Cava, A. M., and Lazoski, C.: Crassostrea gigas in natural oyster banks in southern Brazil, Biol. Invasions, 12, 441–449, https://doi.org/10.1007/s10530-009-9475-7, 2010.
Merkado, G., Holzmann, M., Apothéloz-Perret-Gentil, L., Pawlowski, J., Abdu, U., Almogi-Labin, A., Hyams-Kaphzan, O., Bakhrat, A., and Abramovich, S.: Molecular evidence for Lessepsian invasion of Soritids (larger symbiont bearing benthic foraminifera), PLoS One, 8, e77725, https://doi.org/10.1371/journal.pone.0077725, 2013.
Miller, A. W., Minton, M. S., and Ruiz, G. M.: Geographic limitations and regional differences in ships' ballast water management to reduce marine invasions in the contiguous United States, BioScience, 61, 880–887, https://doi.org/10.1525/bio.2011.61.11.7, 2011.
Mimura, H., Katakura, R., and Ishida, H.: Changes of microbial populations in a ship's ballast water and sediments on a voyage from Japan to Qatar, Mar. Pollut. Bull., 50, 751–757, 2005.
Minchin, D., Floerl, O., Savini, D., and Occhipinti-Ambrogi, A.: Small craft and the spread of exotic species, in: Environmental Pollution, Springer, Netherlands, 99–118, https://doi.org/10.1007/1-4020-4504-2_6, 2006.
Mineur, F., Johnson, M. P., and Maggs, C. A.: Macroalgal introductions by hull fouling on recreational vessels. Seaweeds and sailors, Environ. Manage., 42, 667–676, https://doi.org/10.1007/s00267-008-9185-4, 2008.
Morishima, M. and Chiji, M.: Foraminiferal thanatocoenoses of Akkeshi Bay and its vicinity, Memoirs of the College of Science, University of Kyoto, Series B, 10, 113–117, 1951.
Moss, J. A., McCurry, C., Schwing, P., Jeffrey, W. H., Romero, I. C., Hollander, D. J., and Snyder, R. A.: Molecular characterization of benthic foraminifera communities from the Northeastern Gulf of Mexico shelf and slope following the Deepwater Horizon event, Deep-Sea Res. Pt. I, 115, 1–9, https://doi.org/10.1016/j.dsr.2016.04.010, 2016.
Murphy, K. R., Ritz, D., and Hewitt, C. L.: Heterogeneous zooplankton distribution in a ship's ballast tanks, J. Plankton Res., 24, 729–734, 2002.
Murray, C. C., Pakhomov, E. A., and Therriault, T. W.: Recreational boating: a large unregulated vector transporting marine invasive species, Divers. Distrib., 17, 1161–1172, https://doi.org/10.1111/j.1472-4642.2011.00798.x, 2011.
Murray, C. C., Gartner, H., Gregr, E. J., Chan, K., Pakhomov, E., and Therriault, T. W.: Spatial distribution of marine invasive species: environmental, demographic and vector drivers, Divers. Distrib., 20, 824–836, https://doi.org/10.1111/ddi.12215, 2014.
Murray, J. W.: Ecology and distribution of benthic foraminifera. in: Biology of Foraminifera, edited by: Lee, J. J., Faber, W. W., Anderson, O. R., and Pawlowski, J., Academic Press, 221–254, 1991.
Nagy, J. and Berge, S. H.: Micropalaeontological evidence of brackish water conditions during deposition of the Knorringfjellet Formation, Late Triassic-Early Jurassic, Spitsbergen, Polar Res., 27, 413–427, 2008.
Nagy, J., Pilskog, B., and Wilhelmsen, R.: Facies controlled distribution of foraminifera in the Jurassic North Sea Basin, in: Paleoecology, biostratigraphy, paleoceanography and taxonomy of agglutinated foraminifera, edited by: Hemleben, C., Kaminski, M. A., Kuhnt, W., and Scott, D. B., Dordrecht, Kluwer Academic Press, 621–657, ISBN 9780792310419, ISBN 0792310411, 1990.
Nagy, J., Hess, S., and Alve, E.: Environmental significance of foraminiferal assemblages dominated by small-sized Ammodiscus and Trochammina in Triassic and Jurassic delta-influenced deposits, Earth-Sci. Rev., 99, 31–49, 2010.
Nalepa, T. F. and Schloesser, D. W.: Zebra Mussels: Biology, Impacts, and Control, Lewis Publishers, Boca Raton, Florida, 810 pp., ISBN 0873716965, 1993.
Nichols, F. H., Thompson, J. K., and Schemel, L. E.: Remarkable invasion of San Francisco Bay (California, USA) by the Asian clam Potamocorbula amurensis. II. Displacement of a former community, Mar. Ecol. Prog. Ser., 66, 95–101, 1990.
Nittrouer, C. A., Demaster, D. J., McKee, B. A., Cutshall, N. H., and Larsen, I. L.: The effect of sediment mixing on Pb-210 accumulation rates for the Washington Continental Shelf, Mar. Geol., 54, 201–222, 1984.
Nong, D., Warziniack, T., Countryman, A. M., and Grey, E. K.: Melting Arctic sea ice: Implications for nonindigenous species (NIS) spread in the United States, Environ. Sci. Policy, 91, 81–91, https://doi.org/10.1016/j.envsci.2018.10.013, 2019.
NSW Government: Pacific Oysters, https://www.dpi.nsw.gov.au/dpi/bfs/aquatic-biosecurity/aquatic-pests-and-diseases/marine-pests/bivalve-molluscs/pacific-oyster{#}:$\sim$\,:text$=$Pacific Oysters were introduced deliberately,Western Australia eventually died out (last access: 20 May 2025), 2024.
Oakland Seaport: https://www.oaklandseaport.com/business/facts-figures/ (last access: 20 May 2025), 2024.
O'Brien, P., Barrenechea Angeles, I., Cermakova, K., Pawlowski, J., Alve, E., Nordberg, K., and Polovodova Asteman, I.: Assessing environmental quality in a historically polluted fjord: a comparison of benthic foraminiferal eDNA and morphospecies approaches, J. Geophys. Res.-Biogeo., 129, e2023JG007781, https://doi.org/10.1029/2023JG007781, 2024.
O'Brien, P., Schweizer, M., Morin, F., Hylén, A., Robertson, E. K., Hall, P. O. J., and Polovodova Asteman, I.: Foraminiferal diversity across a sharp gradient of fjord oxygenation: insights from metabarcoding and traditional morphology approach, J. Micropalaeontol., https://doi.org/10.2139/ssrn.5304647, 2025.
Oliveira Neto, F. M.: Síntese informativa da produção de moluscos (mexilhões, ostras e vieiras) comercializados em 2007 no Estado de Santa Catarina, http://www.epagri.rct-sc.br (last access: 20 May 2025), 2008.
Oliveira, U. C.: The role of the Brazilian ports in the improvement of the National Ballast Water Management Program according the provisions of the International BallastWater Convention, The United Nations-Nippon Foundation Fellowship Programme 2007–2008, Division for Ocean Affairs and the Law of the Sea, Office of Legal Affairs, the United Nations, New York, 163 pp., http://www.un.org/depts/los/nippon/unnff_programme_home/fellows_pages/fellows_papers/olivera_0708_brazil.pdf (last access: 20 May 2025), 2008.
Parent, B., Barras, C., and Jorissen F.: An optimized method to concentrate living (Rose Bengal-stained) benthic foraminifera from sandy sediments by high density liquids, Mar. Micropaleontol., 144, 1–13, 2018.
Patterson, R. T.: Arcellaceans and foraminifera from Pleistocene Lake Tecopa, California, J. Foramin. Res., 15, 333–343, 1987.
Pavard, J.-C., Bouchet, V. M. P., Richirt, J., Courleux A., Armynot du Châtelet, E., Duong, G., Abraham, R., Pezy, J.-P., Dauvin, J.-C., and Seuront, L.: Preferential presence in harbours confirms the non-indigenous species status of Ammonia confertitesta (foraminifera) in the English Channel, Aquat. Invasions, 18, 351–369, 2023a.
Pavard, J.-C., Richirt, J., Seuront, L., Blanchet, H., Fouet, M. P. A., Humbert, S., Gouillieux, B., Duong, G., and Bouchet V. M. P.: The great shift: the non-indigenous species Ammonia confertitesta (Foraminifera, Rhizaria) outcompete indigenous Ammonia species in the Gironde estuary (France), Estuar. Coast Shelf S., 289, 108378, https://doi.org/10.1016/j.ecss.2023.108378, 2023b.
Pawlowski, J.: Introduction to the molecular systematics of foraminifera, Micropaleontol., 46, 1–12, 2000.
Pawlowski, J. and Holzmann, M.: A plea for DNA barcoding of foraminifera, J. Foramin. Res., 44, 62–67, 2014.
Pianka, E. R.: On r- and k-selection, Am. Natural., 104, 592–597, 1970.
Polovodova Asteman, I., Hanslik, D., and Nordberg, K.: An almost completed pollution-recovery cycle reflected by sediment geochemistry and benthic foraminiferal assemblages in a Swedish–Norwegian Skagerrak fjord, Mar. Pollut. Bull., 95, 126–140, 2015.
Polovodova Asteman, I., Jaffré, E., Olejnik, A., Holzmann, M., McGann, M., Nordberg, K., Pavard, J.-C, Rösel, D., and Schweizer, M.: Leisure boat harbours, hidden alien species, and pollution: a case study of Hinsholmskilen harbour (Gothenburg, Sweden), J. Micropalaeontol., 44, 119–143, https://https://doi.org/10.15194/jm-44-119-2025, 2025.
Polovodova Asteman, I. and Schönfeld, J.: Recent invasion of the foraminifer Nonionella stella Cushman and Moyer, 1930 in northern European waters: evidence from the Skagerrak and its fjords, J. Micropalaeontol., 35, 20–25, https://https://doi.org/10.1144/jmpaleo2015-007, 2016.
Port of Long Beach: Facts at a Glance, https://polb.com/port-info/port-facts-faqs/#facts-at-a-glance (last access: 20 May 2025), 2024.
Pupo, D. V. and Disaró, S. T.: Characterization of Guaraguaçú River (Paraná, Brazil) based on the distribution of foraminiferal and thecamoebian assemblages and sedimentological analysis, FORAMS 2006-International Symposium on Foraminifera, Natal, Brazil. Anuário Inst. Geociências, 29, 434–435, 2006.
Quinterno, P. J.: Distribution of recent foraminifera in central and south San Francisco Bay, Masters Thesis, San Jose State College, San Jose, California, U.S., 83 pp., 1968.
Quinterno, P. J. and Carkin, B. A.: Benthic foraminifers from Prince William Sound, Alaska – one year after the Exxon Valdez oil spill, in: Sediment of Prince William Sound, beach to deep fjord floor, a year after the Exxon Valdez oil spill, edited by: Carlson, P. R., U.S. Geological Survey Open-File Report 91-631, 31–68, https://doi.org/10.3133/ofr91631, 1991.
Radziejewska, T., Gruszka, P., and Rokicka-Praxmajer, J.: A home away from home: A meiobenthic assemblage in a ship's ballast water tank sediment, Oceanologia, 48, 259–265, 2006.
Rao, K. K.: Ecology of Mandovi and Zuari estuaries, Goa: Distribution of foraminiferal assemblages, Indian J. Mar. Sci., 3, 61–66, 1974.
Rao, K. K., Jayalakshmy, K. V., Venugopal, P., Gopalakrishnan, T. C., and Rajagopal, M. D.: Foraminifera from the Chilka Lake on the east coast of India, J. Mar. Biol. Assoc. India, 42, 47–61, 2000.
Reolid, M., Nagy, J., and Rodríquez-Toval, F. J.: Ecostratigraphic trends of Jurassic agglutinated foraminiferal assemblages as a response to sea-level changes in shelf deposits of Svalbard (Norway), Palaeogeogr. Palaeocl., 293, 184–196, https://doi.org/10.1016/j.palaeo.2010.05.019, 2010.
Reolid, M., Nikitenko, B. L., and Glinskikh, L.: Trochammina as opportunist foraminifera in the Lower Jurassic from north Siberia, Polar Res., 33, 21653, https://doi.org/10.3402/polar.v33.21653, 2014.
Resig, J. M.: Recent foraminifera from a landlocked Hawaiian lake, J. Foramin. Res., 4, 9–76, 1974.
Revets, S. A.: Foraminifera of Leschenault Inlet, J. Roy. Soc. Wes. Austral., 83, 365–375, 2000.
Richards, A. F.: Transpacific distribution of floating pumice from Isla San Benedicto, Mexico, Deep-Sea Res., 5, 29–35, 1958.
Richirt, J., Schweitzer, M., Bouchet, V. M. P., Mouret, A., Quinchard, S., and Jorissen, F.: Morphological distinction of three Ammonia phylotypes occurring along European coasts, J. Foramin. Res., 49, 79–93, 2019.
Richirt, J., Schweizer, M., Mouret, A., Quinchard, S., Saad, S. A., Bouchet, V. M. P., Wade, C. M., and Jorissen, F. J.: Biogeographic distribution of three phylotypes (T1, T2 and T6) of Ammonia (foraminifera, Rhizaria) around Great Britain: new insights from combined molecular and morphological recognition, J. Micropalaeontol., 40, 61–74, https://doi.org/10.5194/jm-40-61-2021, 2021.
Riedel, F., Kossler, K., Tarasov, P., and Wünnemann, B.: A study on Holocene foraminifera from the Aral Sea and West Siberian lakes and its implication for migration pathways, Quaternary Int., 229, 105–111, https://doi.org/10.1016/j.quaint.2010.03.009, 2011.
Rodrigues, A. R., Díaz, T. L. and Pellizari, V. H.: Living foraminifera in a Brazilian subtropical coastal environment (Flamengo Inlet, Ubtuba, São Paulo State-Brazil), in: Approaches to Study Living Foraminifera, edited by: Kitazato, H. and Bernhard, J. M.,Environ. Science and Engineering, Japan, Springer, 195–227, https://doi.org/10.1007/978-4-431-54388-6_11, 2014.
Ross, B. J. and Hallock, P.: Dormancy in the foraminifera: a review, J. Foramin. Res., 45, 358–368, https://doi.org/10.2113/gsjfr.46.4.358, 2016.
Rowlands, M.: Substrata preference in foraminifera of fouling communities in Moorea, French Polynesia, UC Berkeley, UCB Moorea Class, Biology and Geomorphology of Tropical Islands, Student Research Papers, Fall 2007, https://escholarship.org/uc/item/1n31w1ck (last access: 20 May 2025), 2007.
Ruiz, G. M., Carlton, J. T., Grosholz, E. D., and Hines, A. H.: Global invasions of marine and estuarine habitats by non-indigenous species: mechanisms, extent, and consequences, Integr. Comp. Biol., 37, 621–632, 1997.
Ruiz, G. M., Fofonoff, P. W., Carlton, J. T., Wonham, M. J., and Hines, A. H.: Invasion of coastal marine communities in North America: Apparent patterns, processes, and biases, Annu. Rev. Ecol. Syst., 31, 441–480, 2000.
Ruiz, G. M., Fofonoff, P. W., Steves, B., Foss, S. F., and Shiba, S. N.: Marine invasion history and vector analysis of California: A hotspot for western North America, Divers. Distrib., 17, 362–373, https://doi.org/10.1111/j.1472-4642.2011.00742.x, 2011.
Ruiz, G. and Hewitt, C.: Latitudinal patterns of biological invasions in marine ecosystems: A polar perspective, in: Smithsonian at the poles: Contributions to international polar year science, edited by: Krupnik, I., Lang, M. A., and Miller, S. E., Smithsonian Institution Scholarly Press, Washington, D.C., 347–358, ISBN 978-0-9788460-1-5, 2009.
Sanches, T. M.: Distribuição dos foraminíferos recentes na região de Ubatuba, São Paulo. Dissertação de mestrado, Universidade de São Paulo, Instituto Oceanográfico, São Paulo, Brazil, Thesis, 110 pp., unpublished, 1992.
Santschi, P. H., Guo, L., Asbill, S., Allison, M., Kepple, A. B., and Wen, L.-S.: Accumulation rates and sources of sediments and organic carbon on the Palos Verdes shelf based on radioisotopic tracers (137Cs, 239, 240 Pu, 210 Pb, 234Th, 238U and 14C), Mar. Chem., 73, 125–152, 2001.
Schweizer, M., Polovodova, I., Nikulina, A., and Schönfeld, J.: Molecular identification of Ammonia and Elphidium species (Foraminifera, Rotaliida) from the Kiel Fjord (SW Baltic Sea) with rDNAsequences, Helgoland Mar. Res., 65, 1–10, 2011.
Sculati, E.: Still Hanging On, Bay Nature Magazine, https://baynature.org/article/still-hanging-on/ (last access: 20 May 2025), 2004.
SEARATES: Distances and Times, https://www.searates.com/services/distances-time/ (last access: 20 May 2025), 2019.
Seebens, H., Gastner, M. T., and Blasius, B.: The risk of marine bioinvasion caused by global shipping, Ecol. Lett., 16, 782–790, 2013.
Seebens, H., Blackburn, T. M., Dyer, E. E., Genovesi, P., Hulme, P. E., Jeschke, J. M., Pagad, S., Pyšek, P., Winter, M., Arianoutsou, M., Bacher, S., Blasius, B., Brundu, G., Capinha, C., Celesti-Grapow, L., Dawson, W., Dullinger, S., Fuentes, N., Jäger, H., Kartesz, J., Kenis, M., Kreft, H., Kühn, I., Lenzner, B., Liebhold, A., Mosena, A., Moser, D., Nishino, M., Pearman, D., Pergl, J., Rabitsch, W., Rojas-Sandoval, J., Roques, A., Rorke, S., Rossinelli, S., Roy, H. E., Scalera, R., Schindler, S., Štajerová, K., Tokarska-Guzik, B., van Kleunen, M., Walker, K., Weigelt, P., Yamanaka, T., and Essl, F: No saturation in the accumulation of alien species worldwide, Nat. Commun., 8, 14435, https://doi.org/10.1038/ncomms14435, 2017.
Seibold, I.: Preliminary report on the transport of foraminifera into Cochin backwater, J. Mar. Biol. Ass. India, 14, 452–455, 1974.
Silva, J. B. and Duleba, W.: Comparação entre as assinaturas tafonômicas de associações de foraminíferos subfósseis das enseadas do Flamengo e da Fortaleza, São Paulo, Brasil, Rev. Bras. Paleontolog., 16, 263–282, 2013.
Slater, R. A.: Sedimentary environments in Suisun Bay, California. Masters Thesis, University of Southern California, Los Angeles, California, U.S., 104 pp., 1965.
Sjoerdsma, P. G. and Van der Zwaan, G. J.: Simulating the effect of changing organic flux and oxygen content on the distribution of benthic foraminifera, Mar. Micropaleontol., 19, 163–180, 1992.
Smith, L. D., Wonham, M. J., McCann, L. D., Reid, D. M., and Carlton, J. T.: Shipping Study II. Biological invasions by non-indigenous species in United States waters: Quantifying the role of ballast water and sediments. Parts I and II. Report No. CG-D-02-97, U.S. Coast Guard, Washington, D.C., 97 pp., https://apps.dtic.mil/sti/tr/pdf/ADA321543.pdf (last access: 20 May 2025), 1996.
Smith, L. D., Wonham, M. J., McCann, L., Ruiz, G. M., Hines, A. H., and Carlton, J. T.: Invasion pressure to a ballast-flooded estuary and an assessment of inoculant survival, Bio. Invasions, 1, 67–87, 1999.
Steele, E. N.: The Immigrant Oyster (Ostrea Gigas) Now Known as the Pacific Oyster, 169 pp., https://marine-aquaculture.extension.org/wp-content/uploads/2019/05/The-Immigrant-Oyster-the-Pacific-Oyster.pdf (last access: 20 May 2025), 1964.
Steffen, W., Broadgate, W., Deutsch, L., Gaffney, O., and Ludwig, C.: The trajectory of the Anthropocene: The Great Acceleration, Anthropocene Rev., 2, 81–98, 2015.
Strand, Å., Blanda, E., Bodvin, T., Davids, J. K., Jensen, L. F., Holm-Hansen, T. H., Jelmert, A., Lindegarth, S., Mortensen, S., Moy, F. E., Nielsen, P., Norling, P., Nyberg, C., Christensen, H. T., Vismann, B., Holm, M. W., Hansen, B. W., and Dolmer P.: Impact of an icy winter on the Pacific oyster (Crassostrea gigas Thunberg, 1793) populations in Scandinavia, Aqua. Inv., 7, 433–440, https://doi.org/10.3391/ai.2012.7.3.014, 2012.
Stulpinaite, R., Hyams-Kaphzan, O., and Langer, M. R.: Alien and cryptogenic foraminifera in the Mediterranean Sea: A revision of taxa as part of the EU 2020 Marine Strategy Framework Directive, Mediterr. Mar. Sci., 21, 719–758, 2020.
Subías-Barauau, A., Sanchez-Vidal, A., Di Martino, E., and Figuerola, B.: Marine biofouling organisms on beached, buoyant and benthic plastic debris in the Catalan Sea, Mar. Pollut. Bull., 175, 113405, https://doi.org/10.1016/j.marpolbul.2022.113405, 2022.
Swarzenski, P. W.: 210Pb dating, in: Encyclopedia of Scientific Dating Methods, edited by: Rink, W. and Thompson, J., Springer, Dordrecht, 1–11, https://doi.org/10.1007/978-94-007-6326-5, 2014.
Swarzenski, P. W., Baskaran, M., Rosenbauer, R. J., and Orem, W. H.: Historical trace element distribution in sediments from the Mississippi River Delta, Estuar. Coasts, 29, 1094–1107, 2006.
The International Union for Conservation of Nature: Issues Brief, Invasive alien species and climate change, https://www.iucn.org/resources/issues-briefs/invasive-alien-species-and-climate-change (last access: 20 May 2025), 2017.
The Port of Los Angeles: Facts and Figures, https://www.portoflosangeles.org/business/statistics/facts-and-figures (last access: 20 May 2025), 2024.
Thibault de Chanvalon, A., Metzger, E., Mouret, A., Cesbron, F., Knoery, J., Rozuel, E., Launeau, P., Nardelli, M. P., Jorissen, F. J., and Geslin, E.: Two-dimensional distribution of living benthic foraminifera in anoxic sediment layers of an estuarine mudflat (Loire estuary, France), Biogeosciences, 12, 6219–6234, https://doi.org/10.5194/bg-12-6219-2015, 2015.
Thomas, R., Kane, A., and Bierwagen, B. G.: Effects of climate change on aquatic invasive species and implications for management and research, U.S. Environmental Protection Agency Papers No. 51, http://digitalcommons.unl.edu/usepapapers/51 (last access: 20 May 2025), 2008.
Thyrring, J., Blicher, M. E., Sørensen, J. G., Wegeberg, S., and Sejr, M. K.: Rising air temperatures will increase intertidal mussel abundance in the Artic, Mar. Ecol. Prog. Ser., 584, 91–104, https://doi.org/10.3354/meps12369, 2017.
Todd, R.: Foraminifera from Onotoa Atoll, Gilbert Island. U.S. Geological Survey Professional Paper, 354-H, 171–191, 1961.
Toyoda, K. and Kitazato, H.: Paleoenvironmental changes of Yokohama Port since 1870 based on benthic foraminiferal fossils, Ann. Rep. Yokohama Environ. Res. Inst. 116, 11–26, 1995.
Tremblin, C. M. and Haig, D. W.: Evolution of trochamminoids (trochospiral organic-cemented agglutinated Foraminifera): examples from the Lower Permian of Western Australia, J. Foramin. Res., 53, 269–285, https://doi.org./10.2113/gsjfr.53.4.269, 2023.
Tremblin, C. M., Holzmann, M., Parker, J. H., Sadekov, A., and Haig, D. W.: Invasive Japanese foraminifera in a south-west Australian estuary, Mar. Freshwater Res., 73, 328–342, https:// doi.org/10.1071/MF21254, 2021.
Tremblin, C. M. and Walker, J. K.: How did it get here? First record of Trochamminita irregularis, a cosmopolitan estuarine organic-cemented agglutinated foraminifer, in south-west Western Australia, J. Roy. Soc. W. Australia, 108, https://doi.org/10.70880/001c.129191, 2025.
Tsuchiya, M., Kitazato, H., and Pawlowski, J.: Phylogenetic relationship among species of Glabratellidae (Foraminifera) inferred from ribosomal DNA sequences: comparison with morphological and reproductive data, Micropaleontol., 46 (suppl. 1), 13–20, 2000.
Tsuchiya, M., Kitazato, H., and Pawlowski, J.: Analysis of internal transcribed spacer of ribosomal DNA reveals cryptic speciation in Planoglabratella opercularis, J. Foramin. Res., 33, 285–293, https://doi.org/10.2113/0330285, 2003.
Tsuchiya, M., Takahara, K., Aizawa, M., Suzuki-Kanesaki, H., Toyofuku, T., and Kitazato, H.: How has foraminiferal genetic diversity developed? A case study of Planoglabratella opercularis and the species concept inferred from its ecology, distribution, genetics, and breeding behavior, in: Experimental Approaches in Foraminifera: Collection, Maintenance and Experiments, edited by: Kitazato, H., and Bernhard, J., Environmental Science Series Tokyo, Springer, 133–162, https://doi.org/10.1007/978-4-431-54388-6_9, 2014.
Tsujimoto, A., Nomura, R., Yasuhara, M., Yamazaki, H., and Yoshikawa, S.: Impact of eutrophication on shallow marine benthic foraminifers over the last 150 years in Osaka Bay, Japan, Mar. Micropaleontol., 60, 258–268, 2006.
Uchio, T.: Influence of the River Shinano on foraminifera and sediment grain size distributions, Kyoto Univ., Seto Mar. Biol. Lab. Pub., 10, 363–392, 1962.
U.S. EPA (U.S. Environmental Protection Agency) and NPDES (National Pollutant Discharge Elimination System): Vessels, https://www.epa.gov/npdes/vessels-vgp (last access: 20 May 2025), 2017.
U.S. Geological Survey, BRD, CERC, and Texas A&M University: Fate of disposal material and polluted sediment, offshore Honolulu (Coastal and Marine Geology Program), in: Final report on survey of sediment toxicity in the vicinity of Honolulu Hawaii, 18 pp., 1998.
Verna, D. E., Harris, B. P., Holzer, K. K., and Minton, M. S.: Ballast-borne marine invasive species: Exploring the risk to coastal Alaska, USA, Manage. Bio. Invasions, 7, 199–211, https://doi.org/10.3391/mbi.2016.7.2.08, 2016.
Verna, D. E., Minton, M. S., and Ruiz, G. M.: Trade exports predict regional ballast water discharge by ships in San Francisco Bay, Front. Mar. Sci., 8, 638955, https://doi.org/10.3389/fmars.2021.638955, 2021.
Vinogradov, M. Y., Shushkina, E. A., Musayeva, E. I., and Sorokin, P. Y.: A newly acclimated species in the Black Sea: the ctenophore Mnemiopsis leidyi (Ctenophora: Lobata), Oceanology, 29, 220–224, 1989.
Wagner, D. B.: Environmental history of central San Francisco Bay with emphasis on foraminiferal paleontology and clay mineralogy, Doctoral Dissertation, University of California, Berkeley, California, U.S., 274 pp., 1978.
Ware, C., Berge, J., Jelmert, A., Olsen, S. M., Pellissier, L., Wisz, M., Kriticos, D., Smenov, G., Kwasniewski, S., and Alsos, I.: Biological introduction risks from shipping in a warming Arctic, J. Appl. Ecol., 53, 340–349, https://doi.org/10.1111/1365-2664.12566, 2016.
Waters, R., Haigh, N., Whyte, J. N. C., and Levings, C.: Synoptic investigation for algae in ballast water and sediments of ships using selected British Columbia ports, Canadian Data Report of Fisheries and Aquatic Sciences, 1083, 22 pp., 2001.
Weiner, A. K. M., Sehein, T., Cote-L'Heureux, A., Sleith, R. S., Greco, M., Malekshahi, C., Ryan-Embry, C., Ostriker, N., and Katz, L.: Single-cell transcriptomics supports presence of cryptic species and reveals low levels of population genetic diversity in two testate amoebae morphospecies with large population sizes, Evolution, 77, 2472–2483, https://doi.org/10.1093/evolut/qpad158, 2023.
Weinmann, A. E. and Goldstein, S. T.: Landward-directed dispersal of benthic foraminiferal propagules at two shallow-water sites in the Doboy Sound area (Georgia, U.S.A.), J. Foramin. Res., 47, 325–336, https://doi.org/10.2113/gsjfr.47.4.325, 2017.
Weinmann, A. E., Rodder, D., Lotters, S., and Langer, M. R.: Traveling through time: The past, present and future biogeographic range of the invasive foraminifera Amphistegina spp. in the Mediterranean Sea, Mar. Micropaleontol., 105, 30–39, 2013.
Wheatcroft, R. A. and Sommerfield, C. K.: River sediment flux and shelf sediment accumulation rates on the Pacific Northwest margin, Cont. Shelf Res., 25, 311–332, 2005.
Wiesner, H.: Notizen über die Fauna der Adria bei Rovigno. VI. Foraminifera von dem Sandgrunde der Bucht S. Pelagio bei Rovigno, in 3 m Tiefe, Zoo. Anz., 37, 478–480, 1911.
Williams, M., Leinfelder, R., Barnosky, A. D., Head, M. J., McCarthy, F., Cearreta, A., Himson, S., Holmes, R., Waters, C., Zalasiewicz, J., Turner, S., McGann, M., Pilkington, P. M., Berrio, J. C., Wilkinson, I. P., Kaiser, J., Hadly, E. A., Stegner, M. A., Delong, K. L., and Zinke, J.: Planetary-scale change to the biosphere signalled by global species translocations can be used to identify the Anthropocene, Palaeontology, 65, e12688m, https://doi.org/10.1111/pala.12618, 2022.
Williams, R. J., Griffiths, F. B., Van der Wal, E. J., and Kelly, J.: Cargo vessel ballast water as a vector for the transport of non-indigenous marine species, Estuar. Coast. Shelf S., 26, 409–420, 1988.
Williams, S. P.: Oysters, Bay Crossings, https://www.baycrossings.com/oysters/ (last access: 20 May 2025), 2005.
Winston, J. E., Gregory, M. R., and Stevens, C. M.: Encrusters, epibionts, and other biota associated with pelagic plastics: A review of biogeographical, environmental, and conservation issues, in: Marine Debris: Sources, Impacts and Solutions, edited by: Coe, J. M. and Rogers, D. B., New York, Springer, 81–97, ISBN 461384885, 1997.
Wrange, A. L., Valero, J., Harkestad, L. S., Strand, Ø., Lindegarth, S., Christensen, H. T., and Mortensen, S.: Massive settlements of the Pacific oyster, Crassostrea gigas, in Scandinavia, Biol. Invasions, 12, 1145–1152, 2010.
Zheng, S. and Fu, Z.: The agglutinated foraminifera of the Bohai Sea and Huanghai Sea, in: Studies in Benthic Foraminifera, edited by: Takayanagi, Y. and Saito, T., BENTHOS '90, 183–197, 1990.