the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Assessment of anthropogenic metal pollution in Bagnoli (Gulf of Naples) through changes in foraminiferal assemblages and shell chemistry
Sergio Balzano
Matthias Nagy
Petra Heinz
Daniela Gruber
Katy Schmidt
Martin Stockhausen
Thilo Hofmann
Michael Lintner
Chemical pollutants, such as heavy metals, are a major threat to marine ecology and biodiversity in the Mediterranean Sea. The Gulf of Naples plays a crucial role in risk assessment and mitigation of waste contamination in the area, as severe anthropogenic pressure originates from local urban and industrial areas and intense maritime traffic. The now defunct ILVA steel plant in Bagnoli, constructed between 1905 and 1910, was a leading contributor of metal pollution (such as iron, lead, and zinc) in the Gulf of Naples until its shutdown in 1990. In order to evaluate the potentially long-lasting impact of this industrial activity on local benthic foraminiferal communities (living and dead) and the incorporation of metals in benthic foraminifera shells, a contaminated sediment sample near the former steel plant (Site A) was analyzed, and the results were compared to a less impacted sample approximately 1.85 km apart (Site B); inductively coupled plasma mass spectrometry (ICP-MS) and inductively coupled plasma optical emission spectrometry (ICP-OES) revealed exceptionally high levels of metals in the sediment samples taken in close proximity to the former steel plant. Foraminifera community analysis via stereo microscopy and scanning electron microscopy (SEM) concluded slightly lower biodiversity indices and a lower abundance of living foraminifera in the sediment close to the steel plant. Energy-dispersive X-ray spectroscopy (EDX) was utilized to determine concentrations of iron within foraminiferal tests and established that all analyzed specimens from sampling site A had elevated quantities of iron in their tests, compared to individuals from sampling site B. Based on the findings of this investigation, the metal pollution emitted by the former steel mill is still impacting foraminiferal assemblages and individuals to this day. However, the complex interactions of anthropogenic toxins, benthic microorganisms, and the environment are not fully unraveled yet and require further analysis.
- Article
(5386 KB) - Full-text XML
- BibTeX
- EndNote
Shallow coastal marine environments, as the main destination of terrestrial runoff, are particularly endangered by various types of industrial and domestic waste (Samir, 2000; Yanko et al., 2003; Le Cadre and Debenay, 2006; Sreenivasulu et al., 2017; Fajemila et al., 2022; El-Kahawy and Mabrouk, 2023). Among all anthropogenic pollutants, some metals are exceptionally harmful to organisms due to their toxicity, persistence, and bioaccumulation (Frontalini et al., 2018). Although most heavy metals are essential for several biological purposes at low concentrations, they may be toxic to marine biota once a specific threshold is met (Frontalini and Coccioni, 2008).
Apart from agricultural activity, one of the largest sources of anthropogenic toxic metal pollution is the metallurgical industry, which encompasses mining, smelting, metal finishing, and other procedures. Even so, not only metallic ores are responsible for deleterious emissions of toxic metals, as coal combustion is also an important contributing factor. Depending on parameters such as composition of the coal, burning conditions, handling of by-products, and emission control efficiency, toxic metals and metalloids, such as arsenic, cadmium, molybdenum, selenium, and zinc, can be detected at varying concentrations within coal residues (Bradl, 2005). Both industrial coal combustion and resulting coal residue directly affect nearby coastal environments, potentially leading to trace element concentrations beyond regulatory safety limits (Kok et al., 2019).
Industrial activities emit toxic metals into the environment in a variety of forms: gaseous, particulate, aqueous, or even solid (Bradl, 2005). Mobility and behavior of these pollutants depend on both chemical and biological circumstances (Yanko et al., 2003; Bradl, 2005; Gao et al., 2023): acidic waters, commonly generated by the oxidation of pyrite (FeS2), lead to increased mobility and solubility of toxic metals, which consequently can be adsorbed onto algae and accumulate within different trophic levels throughout the food web (Bradl, 2005). Ocean acidification, particularly in coastal marine settings, is another factor increasing the mobility and ecological damage potential of toxic metals (Gao et al., 2023). Benthic organisms can affect the chemical properties of water, sediments, and pollutants via complex biological processes, eventually increasing or decreasing the toxicity of anthropogenic waste (Yanko et al., 2003). At the same time, bottom-dwelling marine organisms are especially exposed to toxic metal pollution, as anthropogenic contaminants accumulate within the marine sediments they inhabit (Andersen et al., 2019).
Due to their abundance in nearly all marine and transitional habitats, short reproductive cycles, high species diversity, long-term preservation as mineralized tests, and potential for statistical evaluation due to their small size and pervasiveness in marine sediments, foraminifera (marine single-celled organisms) are widely used as bioindicators for the environmental monitoring of pollution (e.g., Samir, 2000; Bouchet et al., 2012; Alve et al., 2016; Sreenivasulu et al., 2017), including metal pollution (e.g., Frontalini et al., 2009; Boehnert et al., 2020; El-Kahawy and Mabrouk, 2023). As such, they are commonly analyzed in terms of assemblage structure, shell chemistry, ultrastructure, pyritization, reproductive capability, and prolocular and test morphology (e.g., Frontalini et al., 2009). Additionally, ecological indices have been developed to assess the ecological quality status using benthic foraminifera. Examples include the Ammonia-Elphidium Foraminiferal Index (Sen Gupta et al., 1996; Sen Gupta and Platon, 2006) and the Foram-AMBI index (Alve et al., 2016; Jorissen et al., 2018). These indices primarily rely on the sensitivity or tolerance of species to organic matter enrichment, often associated with increased oxygen consumption (Alve et al., 2016).
The vast majority of foraminifera are surrounded by a protective shell (test), which can be organic (non-mineralized), agglutinated (consisting of cemented sediment particles), or composed of biomineralized calcium carbonate (either hyaline low-magnesium calcite, miliolid high-magnesium calcite, or aragonite) or even opaline silica (Goldstein, 2003; Hallock, 2003; Sen Gupta, 2003). Foraminifera inhabit all types of marine environments and have a high fossilization potential, making them an important tool for paleoecological and paleostratigraphic purposes (Pawlowski, 2012). The distribution, diversity, and abundance of foraminifera are driven primarily by abiotic factors, such as temperature, light intensity, dissolved oxygen, nutrient concentration, type of substrate, and salinity (Pawlowski, 2012; Binczewska et al., 2015). In general, some taxa demonstrate more sensitivity towards certain environmental factors, while others have broader tolerance ranges (Murray, 1991).
Foraminiferal morphological irregularities such as test deformations were already described two centuries ago (Carpenter, 1856) and can be found in both fossil and recent specimens (Geslin et al., 2000). These test abnormalities are a general occurrence among all benthic foraminifera, regardless of taxonomy, test morphotype, and mode of life. While some deformities are spontaneous, others are linked to external conditions (Yanko et al., 1998): test deformations can be caused by numerous environmental aspects, such as fluctuations in salinity and temperature, inadequate food and light, substrate type, pH level, hydrodynamics, and marine pollution (Yanko et al., 1998; Geslin et al., 2000; El-Kahawy and Mabrouk, 2023).
However, a drastic increase in abnormal tests can be measured in anthropogenically polluted marine habitats (Yanko et al., 1998; Stubbles, 1999; Geslin et al., 2000; Samir, 2000; Le Cadre and Debenay, 2006; Frontalini et al., 2009; Hart et al., 2014; Sreenivasulu et al., 2017), making foraminifera outstanding proxies for sewage, liquid hydrocarbons, and heavy metals (Yanko et al., 1998; Le Cadre and Debenay, 2006). Foraminifera are subjected to both biological and mineralogical alterations induced by toxic metal exposure: Nardelli et al. (2016) described disorganized calcite crystals on the internal test surface of the miliolid Pseudotriloculina rotunda, resulting from the incorporation of zinc into the crystal lattice. Despite these findings, no associated test deformations could be identified, implying a different underlying mechanism (Nardelli et al., 2016). Overall, the degree to which heavy metals are integrated into foraminiferal tests is proportional to the corresponding concentration in surrounding waters (Boehnert et al., 2020; Schmidt et al., 2022).
From a biological perspective, abnormal foraminiferal tests can be explained through cytological defense mechanisms (Frontalini et al., 2018; Boehnert et al., 2020). As a response to zinc, lead, and mercury exposure, a rise in degradation vacuoles, residual bodies, and lipid droplets, along with a thickening of the inner organic lining, can be observed (Frontalini et al., 2018), perhaps in order to mitigate the effects of toxic-metal-induced mitochondrial degeneration, inhibited metabolism and protein synthesis, and disrupted membrane permeability (Yanko et al., 1998; Frontalini et al., 2018; Boehnert et al., 2020; Lintner et al., 2021). It is important to note that not all metals influence living foraminifera equally; pseudopodial activity, and thus food uptake, is critically limited by elevated levels of copper and zinc, whereas higher amounts of lead do not induce such impairment (Lintner et al., 2021, Bubl et al., 2024). Additionally, the quantity of toxic metal exposure is also a necessary factor to consider: as an essential trace metal, zinc stimulates metabolic processes of Elphidium excavatum at lower concentrations, while higher concentrations are toxic to the foraminifera, as assessed by Lintner et al. (2021).
As described by the European Environment Agency in 2019, more than 87 % of the Mediterranean Sea is classified as problematic in the context of environmental pollution. Heavy metals are the most prevalent type of contaminant encountered in the assessed samples (Andersen et al., 2019). The now-defunct steel manufacturing plant in Bagnoli was a leading contributor of anthropogenic pollution in the Gulf of Naples. The factory, located on the outskirts of Naples, was constructed by ILVA between 1905 and 1910 and became one of the most important sites for steel production in Italy (Romano et al., 2009). Until the shutdown of the plant in 1990, the locality and especially the natural coastline experienced significant changes: in 1930, two long piers were built, allowing the docking of large-tonnage ships. Raw materials, such as iron ore and fossil coal, were unloaded at the northern pier, whereas the southern pier was used to load manufactured products onto cargo vessels (Romano et al., 2004, 2009). The year 1935 saw a change in water circulation and, consequently, sediment distribution in the surrounding area as a stone bridge connecting the nearby isle of Nisida with the mainland was established (Romano et al., 2004). As it was in need of more space for coal storage and industrial buildings, the marine area between both piers was partially filled with contaminated soil from the steel mill between 1962 and 1964 (Romano et al., 2004, 2009).
During operation of the industrial plant, exceptionally high concentrations of heavy metals, such as silver, copper, lead, zinc, nickel, cobalt, cadmium, and mercury, were measured in nearby marine sediments, particularly between the piers. At the same time, unusually high sedimentation rates of 0.4 cm yr−1 were determined in front of the steel plant, implying a large anthropogenic influx of grains from the industrial site (Romano et al., 2009). Based on the sedimentation rates, it is estimated that the first input of pollutants occurred in the 1950s (Romano et al., 2018), the decade before steel production in Bagnoli would reach its maximum in 1960 (Romano et al., 2009), followed by a peak in contamination in the time interval 1950–1980 (Romano et al., 2018). Furthermore, the soil of the brownfield site is contaminated with copper, chromium, lead, zinc, and nickel (Romano et al., 2009).
However, the former ILVA steel mill was not the only source of toxic pollutants in the Gulf of Naples. The surrounding industrial area was occupied by companies manufacturing asbestos materials (Eternit), fertilizers (Federconsorzi), and cement (Cementir) (Albanese et al., 2010). The industrial area is also situated at the northern edge of a volcanic caldera belonging to the Campi Flegrei volcano–tectonic system (Romano et al., 2004), and previous studies have unveiled a correlation between submarine hydrothermal springs and certain toxic metals in marine sediments (Albanese et al., 2010).
Romano et al. (2009) recognized a strong correlation between heavy metal pollution, sediment composition, foraminiferal deformities, abundance of pollution-tolerant taxa, and proximity to the steel plant dock. Assemblage and geochemical analysis conducted in the nearby Naples harbor revealed elevated levels of toxic metals and simultaneously decreased foraminiferal density and diversity, partially culminating in the complete absence of foraminifera (Ferraro et al., 2009; Ferraro et al., 2006).
This investigation employs a 3-fold approach in order to evaluate the potentially long-lasting effects of industrial metal pollution on foraminifera. Geochemical analysis of the contaminated sediment will provide information about the current degree of metal pollution of two sites: one sampling area roughly 1.85 km apart from the steel plant and one site at the same polluted location previously described by other studies. Furthermore, the foraminiferal assemblages in the contaminated and uncontaminated sampling areas will unravel the long-term impact of anthropogenic metal pollution on benthic foraminifera and coastal marine ecosystems. Ultimately, the chemical analysis of individual foraminifera tests will aid the understanding of (species-)specific responses to toxic metal influx and may help uncover underlying mechanisms for the aforementioned distribution of foraminifera in contaminated and uncontaminated habitats.
2.1 Sampling and preparation
A single sample (constituted of three replicates) was taken from two sampling sites (A, B) in the Gulf of Naples (approximately 1.85 km apart; Fig. 1) in March 2022. For each replicate, the topmost 1 cm of an area (30×30 cm) was sampled by divers. One sampling point (Site A) is in direct proximity to the former steel mill, located between the two piers (40.807114° N, 14.164777° E), while the other serves as a control site (40.794625° N, 14.179893° E) (Site B), physically separated from the steel plant dock by a stone bridge between the isle of Nisida and the mainland. Both sampling sites are located in areas with comparable environmental parameters (depth, salinity, temperature, distance from the coast). The salinity, pH value, and temperature were measured directly on site using suitable probes from Carl Roth.
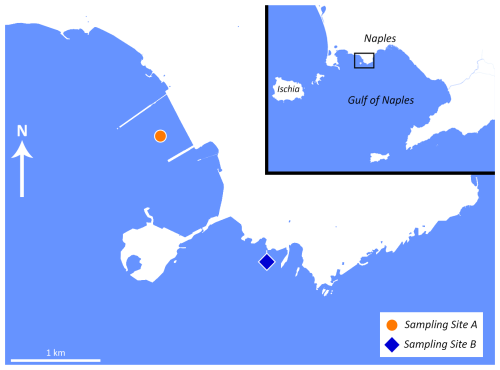
Figure 1Map of the sampling locations. Site A is marked with an orange dot, and Site B is marked with a blue square. Map adapted from © OpenStreetMap contributors 2025. Distributed under the Open Data Commons Open Database License (ODbL) v1.0.
All sediment samples were preserved in 96 % ethanol after collection to permit further analysis (Schönfeld et al., 2012). Afterwards, the samples were stained with rose bengal solution (2 g L−1 in 96 % ethanol, in accordance with Schönfeld et al., 2012) for 2 d (reduced exposure time to rose bengal solution as the samples had already been preserved in ethanol for several weeks) and subsequently wet-sieved through 125 and 63 µm mesh sieves. Lastly, the resulting 125 µm size fraction was dried at 50 °C for several days and split into subsamples. The splits had to contain at least 300 identifiable benthic foraminifera individuals to be considered for further analyses (Schönfeld et al., 2012).
2.2 Geochemical and grain size analysis
To analyze the geochemistry of the sediments, the replicates were split separately using a suitable sediment splitter until a mass of approximately 1 g of sediment was obtained. This aliquot was then ground to powder using a ball mill to obtain the required amount of sediment for geochemical analysis. The sediment samples were digested on a hotplate in a multi-step process: firstly, aqua regia (1.5 mL HCl (30 %) and 0.5 mL HNO3 (65 %), both in analytically pure quality) was added to the sample and evaporated at 140 °C until dry. Afterwards, 0.5 mL of HCl (30 %) and 1.5 mL of HNO3 (65 %) were added to the residue, and the resulting mixture was heated at 120 °C for 12 hours. The samples were then dried at 120 °C and taken up in 2 mL HNO3 (65 %). Components that could not be dissolved with this process (e.g., silicates) were filtered out.
Metal concentrations were then measured using inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7900 Single Quad ICP-MS in helium mode) and inductively coupled plasma optical emission spectrometry (ICP-OES; Agilent 5110 in axial mode). To mitigate matrix effects, ICP-MS measurements were conducted with an activated collision cell in helium mode. Calibration standards for both ICP-MS and ICP-OES were prepared using matrices tailored to match the sample matrix composition. Furthermore, samples were appropriately diluted in order to minimize potential matrix effects. Variations in instrument response due to matrix effects were corrected by employing rhodium as an internal standard.
Energy-dispersive X-ray spectroscopy (EDX; JEOL IT300 in low vacuum mode, EDAX AMETEK detector, 16 dot-mappings per individual at 512×400 resolution and 200 µs dwell time) was used to analyze the chemical composition of the foraminifera tests and to distinguish areas with elevated levels of foreign ions therein, which may correspond with test deformations. The aforementioned parameters were chosen in order to reduce measurement time and sample heating while also ensuring satisfactory image quality.
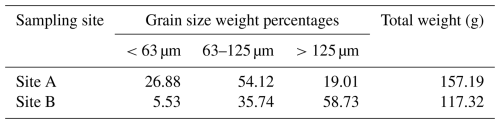
For grain size analysis, sediment preserved in ethanol was dried at 50 °C for 3 d and weighed (total mass). The dry sediment was then wet-washed through a 125 and 63 µm sieve, and the two resulting fractions (>125 and 125–63 µm) were collected separately. After another drying phase at 50 °C for 3 d, the individual fractions were weighed, and the proportions were calculated. The amount of fine fraction (<63 µm) was obtained by subtracting the two fractions from the total mass.
2.3 Foraminiferal analysis
Taxonomical information was gathered via stereo microscopy (Motic SMZ-168) of foraminifera isolated from the sediment samples, supported by scanning electron microscopy (SEM; JEOL IT300 in low vacuum mode, JEOL JSM-6400 and TESCAN Vega 4 GMU in high vacuum mode). The latter was used to create SEM plates of dominant species and whenever stereo microscopy proved to be insufficient for taxonomical identification. Since the sediment was previously stained with rose bengal, foraminifera that were alive at the time of sampling can be visually distinguished by their color (rose bengal stains cytoplasm of living individuals) from those that were already dead (Schönfeld et al., 2012). These quantitative and qualitative evaluations are necessary to investigate the foraminiferal assemblages present, a possible correlation between the degree of pollution and the abundance of occurring specimen and the ratio of alive to dead individuals.
2.4 Statistical analysis
The program PAST 4.12b was used for statistical evaluation of the samples; individual rarefaction curves were applied to determine the minimal number of foraminifera needed in each sample for statistically relevant conclusions, and diversity indices were calculated in order to define alpha and beta diversity (Hammer et al., 2001). Alpha diversity indices (e.g., Fisher's alpha index, Shannon–Wiener index, Simpson index) and beta diversity indices (e.g., Morisita's overlap index) are a well-established and widely used metric to evaluate the spatial ecology of sampled foraminifera (Murray, 1991; Samir, 2000; Romano et al., 2009; Stephenson et al., 2015; El-Kahawy and Mabrouk, 2023). Morisita's overlap index is the most appropriate choice among beta diversity indices and is particularly well suited due to its robustness to undersampling (Barwell et al., 2015). In addition, two-way analysis of variance (ANOVA) was performed to examine the differences in metal concentrations between the two sampling sites.
3.1 Ecological analysis
The sea surface temperature at both sampling stations reached 14 °C at the time of collection, with a salinity of 37 each. Sampling site A is situated at 8 m depth, while sampling site B is located 11 m below sea surface. At a pH of 7.5, the water of Site A is more acidic compared to Site B, which has a pH of 8.0.
Regarding grain size distribution of the sediment samples, the composition of the samples varies strongly. Sediment samples gathered near the former steel mill (Site A) contain a higher percentage of silt- and clay-sized grains, whereas the sediment from Site B is dominated by coarser clasts (Table 1).
Table 1Grain size distribution of the sediment samples. Site A is the sampling site between the docks near the steel plant, and Site B is about 1.85 km away.
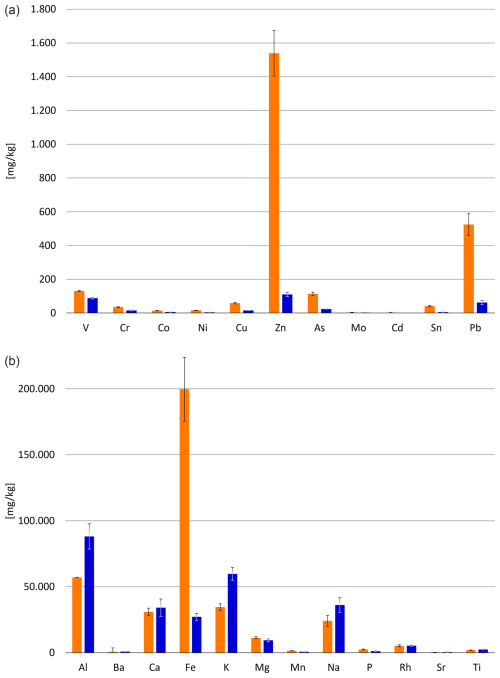
The results of the ICP-MS and ICP-OES measurements show notable differences in chemical contents of the investigated sediments: as shown in Fig. 2, metal concentrations at the decommissioned steel plant dock (Site A) greatly exceed the measured values of the second station (Site B). The concentration of iron at site A is more than 7 times the value of that at Site B (199.728 and 27.206 mg kg−1, respectively), zinc is almost 14 times as prevalent (1.540 and 110 mg kg−1, respectively), and lead occurs at Site A in roughly 8-fold quantities compared to Site B (524 and 63 mg kg−1, respectively). This is further supported by two-way ANOVA, which confirms that the metal content of Site A differs highly significantly (p<0.001) from that of Site B. The underlying assumption of the two-way ANOVA regarding normality was verified via a Shapiro–Wilk test (W p<0.001).
3.2 Foraminiferal assemblages
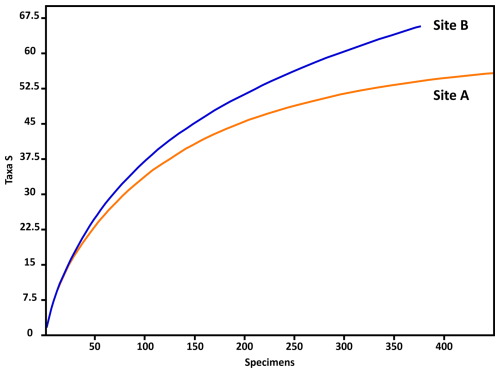
According to the individual rarefaction curves of both samples (Fig. 3), statistically and ecologically significant conclusions can be drawn by extrapolating the total (living and dead) foraminiferal assemblages in the sediment samples to the investigated habitats.
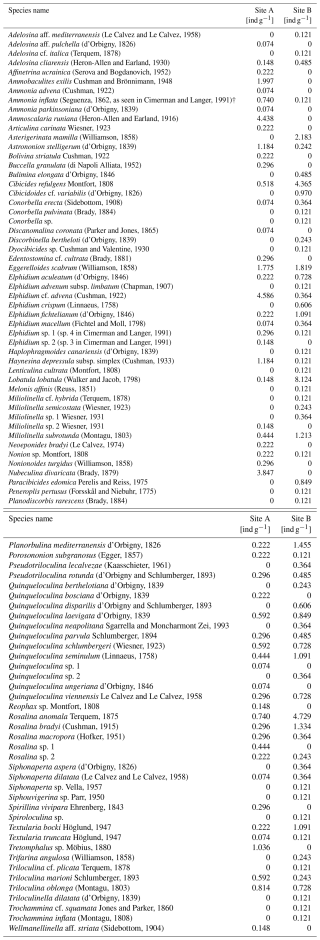
Every discernible foraminiferal species found in the sampled sediments, along with its corresponding abundance, is listed in Table A1 and is publicly available in a database (Plakolm and Nagy, 2025).
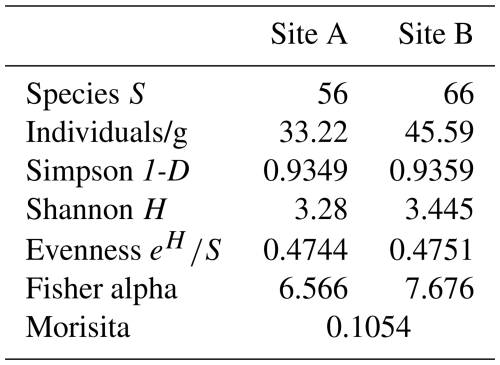
Analysis of foraminiferal components showed that Site A contains 33.22 individuals per gram sediment, which are heterogeneously distributed across 56 species. Site B consists of 45.59 individuals per gram sediment and 66 species.
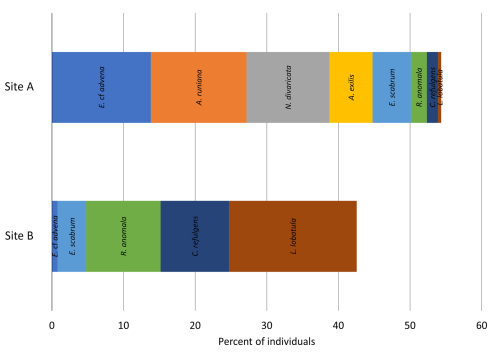
Dominance among the species is defined as an abundance of >5 % of each sampling site's total (living and dead) assemblage. Five species of sampling site A fulfill this criterion: Elphidium cf. advena (13.81 %), Ammoscalaria runiana (13.36 %), Nubeculina divaricata (11.58 %), Ammobaculites exilis (6.01 %), and Eggerelloides scabrum (5.35 %). In this sample, 52.73 % of species and 44.1 % of individuals are hyaline, 36.36 % of species and 29.84 % of individuals are miliolid, and 10.91 % of species and 26.06 % of individuals are agglutinated (Fig. 4). Moreover, 7.13 % of the investigated specimen are considered alive based on their interaction with rose bengal.
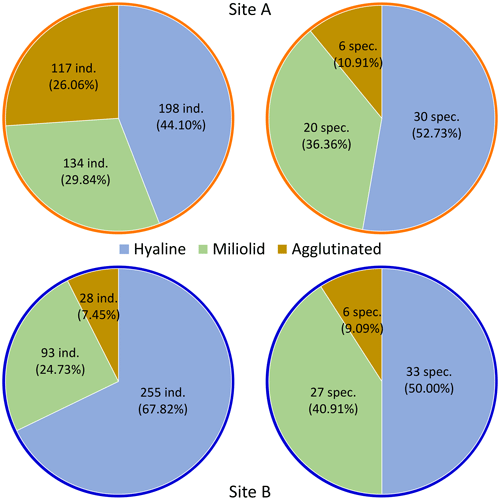
Figure 4Relative and absolute abundance of individuals (Site A n=449; Site B n=376) and species (Site A n=56; Site B n=66), based on test material.
Sediments from Site B are characterized by the dominance of Lobatula lobatula (17.82 %), Rosalina anomala (10.37 %), and Cibicides refulgens (9.57 %). In this sample, 50 % of species and 67.82 % of individuals are hyaline, 40.91 % of species and 24.73 % of individuals are miliolid, and 9.09 % of species and 7.45 % of individuals are agglutinated (Fig. 4). In this sample, 14.89 % of the individuals are considered alive.
Among all species contributing to at least 1 % of their corresponding foraminiferal assemblage (≥5 individuals at Site A and ≥4 individuals at Site B), only one species (Q. seminulum, Site B) has more individuals considered alive than dead (Fig. 5). In both sites, living foraminifera are distributed relatively evenly across different species. However, there are notable differences regarding test materials: while 75.86 % of all living individuals at Site A have a hyaline test and 24.14 % have a miliolid test, only 56.86 % of living foraminifera at Site B are hyaline and 43.14 % are miliolid. Agglutinated foraminifera are not considered for this assessment because their non-transparent tests conceal the (possibly stained) cytoplasm on the inside.
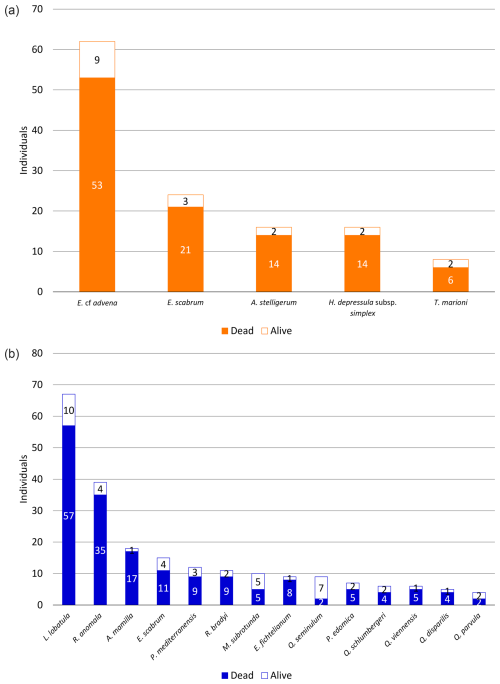
Figure 5Distribution of dead and alive foraminifera across different species sampled at Site A (a) and Site B (b). Only species with individuals contributing to ≥1 % of the corresponding sample and at least one living individual are depicted.
The variation in dominant species between the sampling sites displayed in Figs. 6 and 7 is further emphasized by diversity indices (Table 2). According to the calculated values, both samples have comparable diversities. However, the Shannon–Wiener index (H) shows a slightly higher diversity at Site B (3.445) compared to Site A (3.28), as does Fisher's alpha index (Site B: 7.676; Site A: 6.566). Individuals are slightly more evenly distributed across the species at Site B, as expressed by the evenness (Site B: 0.4751; Site A: 0.4745). Values for the Simpson index reveal slightly more rare species at Site B (Site B: 0.9359; Site A: 0.9349).
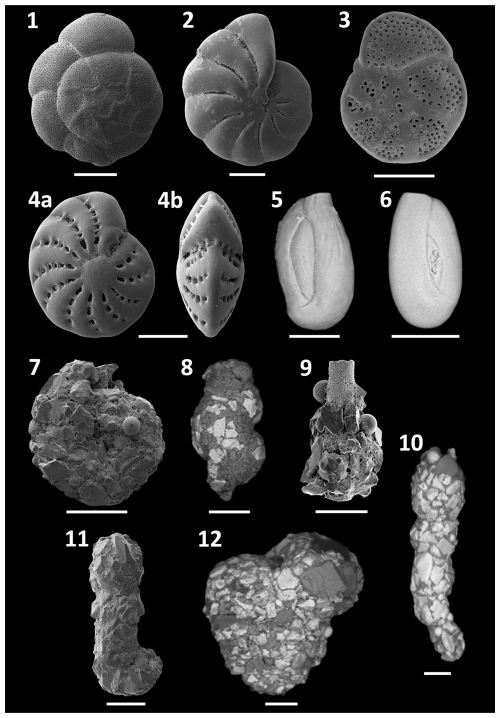
Figure 6SEM plate of dominant and characteristic species found at Site A. (1) Ammonia inflata, (2) Haynesina depressula subsp. simplex, (3) Rosalina bradyi, (4) Elphidium cf. advena (a: side view; b: apertural view), (5) Quinqueloculina parvula, (6) Quinqueloculina seminulum, (7) Ammoscalaria runiana, (8) Eggerelloides scabrum, (9) Nubeculina divaricata, (10) Reophax sp., (11) Ammobaculites exilis, (12) Textularia bocki. All scale bars = 100 µm.
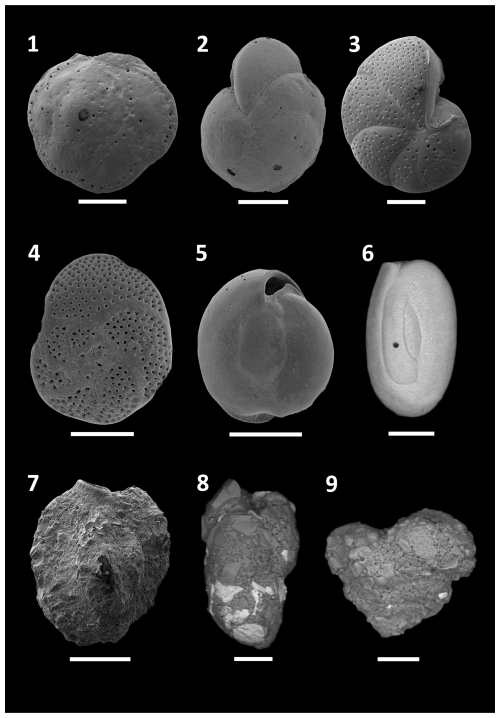
Figure 7SEM plate of dominant and characteristic species found at Site B. (1) Asterigerinata mamilla, (2) Cibicides refulgens, (3) Lobatula lobatula, (4) Rosalina bradyi, (5) Miliolinella subrotunda, (6) Quinqueloculina seminulum, (7) Quinqueloculina berthelotiana, (8) Eggerelloides scabrum, (9) Textularia bocki. All scale bars = 100 µm.
However, Morisita's overlap index indicates little similarity between the two samples. Only 10.54 % of the identified species occur in both samples and in similar proportions. This index is dominated by the most abundant species; thus the numerous rarely occurring species have a lower impact. Nonetheless, it is noteworthy that none of the dominant species are overlapping between the two sampling sites (i.e., are dominant in both samples), indicating two greatly differing assemblage compositions (Fig. 8).
Analysis of representative subsamples for each sampling location (Site A subsample n=108; Site B subsample n=138) uncovered the complete absence of test deformations in miliolid foraminifera and slightly more test deformations occurring in hyaline foraminifera from the subsample Site A: 10.26 % of hyaline individuals from the habitat at Site A display malformed chambers, whereas 6.8 % of hyaline tests from Site B demonstrate such deformations (>2 % implying stressful conditions; Coccioni and Marsili, 2005). The subsamples included no agglutinated foraminifera, as evaluation of test deformations proved unreliable and led to inconsistent results.
3.3 Chemical analysis of foraminiferal tests
EDX analysis of six representative individuals of the species Elphidium cf. advena, Rosalina anomala, Eggerelloides scabrum, Textularia bocki, Quinqueloculina seminulum, and Quinqueloculina parvula for each sample reveals a correlation between increased amounts of iron within foraminiferal tests of all materials (hyaline, miliolid, agglutinated) and higher concentrations of iron in their corresponding environments (as established previously by the results depicted in Fig. 2). Figure 9 illustrates this correlation between iron concentrations in ambient sediment of Site A and the tests of foraminifera inhabiting that sediment compared to Site B.
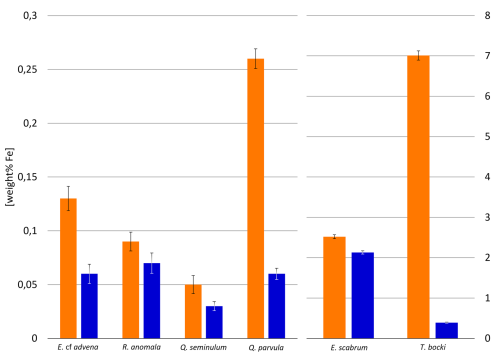
Figure 9Weight percentages of Fe detected in individuals of different species across samples from Site A (orange) and Site B (blue). Error bars represent measurement error during EDX analysis.
Figures 10 and 11 allow visual evaluation of the physical distribution of iron-rich areas within the investigated foraminifera. The hyaline foraminifera of the species Elphidium cf. advena and Rosalina anomala show no distinct pattern of iron distribution in their tests, independent of their sampling area. Individuals of the miliolid species Quinqueloculina seminulum and Quinqueloculina parvula, however, partially portray a distribution pattern: the individual of the species Quinqueloculina parvula found at Site A has a higher density of iron-containing regions of interest (ROIs) along sutures and ridges of the outermost chambers, compared to the remainder of miliolid individuals from both sampling stations (Site A and B). As seen in Fig. 9, the agglutinated individuals representing the species Eggerelloides scabrum and Textularia bocki have relatively high amounts of iron in their tests. According to the ROIs in Figs. 10 and 11, for both species, cemented, dark sediment particles are responsible for the majority of measured iron content. While both examples of Eggerelloides scabrum have comparable distributions of the aforementioned dark sediment particles (and thus ROIs for iron), the analyzed individuals belonging to the species Textularia bocki display vastly different quantities of iron-rich sediment inclusions depending on the sample, resulting in highly contrasting detected weight percentages of iron (Fig. 9). The results of EDX analysis further reveal no correlation between test malformations in hyaline foraminifera and detectable quantities of foreign metal ions in the affected area, regardless of sampling location (e.g., Elphidium cf. advena in Figs. 10 and 11).
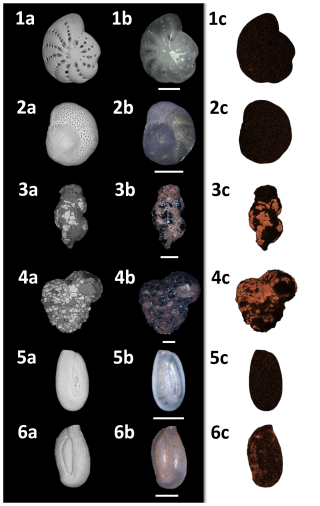
Figure 10SEM/photomicroscope/EDX plate of representative species found at Site A. (1) Elphidium cf. advena, (2) Rosalina anomala, (3) Eggerelloides scabrum, (4) Textularia bocki, (5) Quinqueloculina seminulum, (6) Quinqueloculina parvula. (a) SEM image, (b) photomicroscopic image, (c) EDX regions of interest (ROIs) for iron. All scale bars = 100 µm.
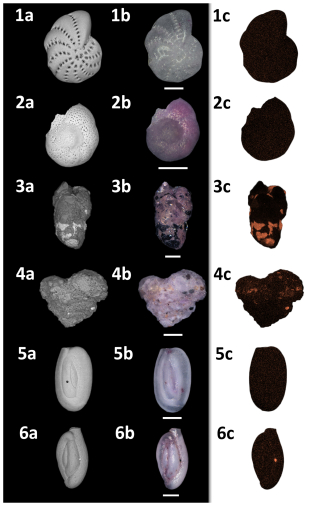
Figure 11SEM/photomicroscope/EDX plate of representative species found at Site B. (1) Elphidium cf. advena, (2) Rosalina anomala, (3) Eggerelloides scabrum, (4) Textularia bocki, (5) Quinqueloculina seminulum, (6) Quinqueloculina parvula. (a) SEM image, (b) photomicroscopic image, (c) EDX regions of interest (ROIs) for iron. All scale bars = 100 µm.
4.1 Differences between sampling locations
Both sampling sites differ significantly in their elemental and grain size composition. Although this difference in sediment grain size distribution was already noted by Romano et al. (2009), it is important to mention that these previous results are not entirely congruent with the current data. While the aforementioned study determined 18.4 % sediment components < 63 µm at the sampling site in close proximity to the steel plant and 2.8 % sediment components < 63 µm at the reference sampling site, the current results are 26.9 % constituents < 63 µm close to the steel plant and 5.5 % constituents < 63 µm at Site B. As reported by Romano et al. (2009), sediment granulometry in Bagnoli Bay is highly variable, and even two points 4–5 m apart might have a different grain size composition, which may also explain the differences between the studies. This naturally leads to an irregular distribution of metals. In the Bay of Bagnoli, metals such as Fe, Zn, Pb, and Mg are found correlative in the same concentrations, whereas metals such as Cu, Hg, and Cd can have site-specific hotspots (Bergamin et al., 2005).
The grain size of seabed sediment is influenced by various factors, most notably local hydrodynamics, which undoubtedly vary between the two sampling sites. Nevertheless, the industrial site contributed an exceptional artificial input of grains during the operational period of the steel plant. Subsequently, these anthropogenic inputs were likely deposited on the seabed in significant quantities, as evidenced by the sedimentation rates (Romano et al., 2009). Consequently, the sediment composition appears to have shifted substantially towards finer grain sizes. This shift may indirectly affect the assemblage composition of benthic foraminifera, as their habitat preferences are closely related to the grain size distribution of the sediment (e.g., Murray, 1991; Romano et al., 2009). Another potential indirect effect of the grain size is the link between certain metal pollutants (such as Pb and Zn) and silty sand fractions (Romano et al., 2008). At this point, it must be mentioned that the difference in the foraminiferal community reported by our study can possibly be in part attributed to the grain size. The grain size differs between Site A and Site B by up to 20 % in fine fraction and is supposed to affect the foraminiferal diversity in that area (Romano et al., 2013). The inherent link between anthropogenic metal pollution and sediment influx at Site A makes the grain size shift yet another aspect and direct consequence of industrial pollution. In conjunction with several anthropogenic alterations of the local coastline (e.g., construction of two piers) and the resulting modification of hydrodynamics, the artificial fine-particle input has a vastly increased potential to remain and accumulate within Site A (e.g., Albanese et al., 2010; Hendriks et al., 2020).
Earlier measurements confirm the increased levels of iron, zinc, and lead in the sediments directly in front of the steel plant: Romano et al. (2009) measured 74 600 mg kg−1 iron, 1523 mg kg−1 zinc, and 465 mg kg−1 lead close to the steel plant. The discrepancy between these values and the current data (199 728 mg kg−1 iron, 1540 mg kg−1 zinc, 524 mg kg−1 lead) implies an increase in heavy metal pollution during the past decades, long after the shutdown of the steel mill. A study in 2019 has shown that even recent deposition of highly contaminated sediment can be found in the region around the mill (Sprovieri et al., 2019). Organic pollutants have also increased in Bagnoli Bay in recent years. The concentration of polycyclic aromatic hydrocarbons (PAHs) is highest precisely between the two docks, correlates with the total organic carbon in the sediment (Sprovieri et al., 2019), and reached concentrations of up to 87 000 µg g−1 sediment dry weight (Passaro et al., 2020). However, as we collected one sample for foraminiferal analysis per site, further studies are necessary to obtain a detailed overview about the current state of pollution.
Zinc and lead, initially part of sulfide minerals within coal and certain ores, are commonly enriched within fly ash and can subsequently be mobilized into surface waters and soils. As opposed to other metals, zinc and lead are only transported as particles, and large amounts of lead are released into the environment by gasoline additives (Bradl, 2005).
Another source of marine zinc and lead pollution is antifouling paint: apart from biocidal organotin compounds (e.g., tributyltin), which are now banned for this purpose, copper-based biocides and zinc-based booster biocides (such as zinc oxide and zinc pyrithione) are widely used in antifouling paint on boats and ships (Jeong et al., 2023). Due to their corrosion resistance and color, lead compounds are also commonly found in marine paint (Jeong et al., 2023). Despite the aforementioned ban on some biocidal additives, previously released metals might have been accumulating in the sediment.
The former Federconsorzi fertilizer production facility, located next to the defunct steel plant, could potentially be yet another point of origin for zinc emission, due to phosphatic fertilizers often containing metals, such as zinc (Bradl, 2005; Albanese et al., 2010). This would partially explain the current elevated levels of toxic metals in the sediments investigated by this study.
All of our findings imply that the area in close proximity to the old steel plant is still severely contaminated with toxic metals and metalloids, which potentially results in a shift in the living microbial community (see Figs. 4 and 8).
4.2 Community shift between Site A and Site B
The assemblage compositions of the two sampling sites exhibit large differences; none of the dominant species of each sample overlap in their abundance, and neither do multiple rare species; thus the Morisita similarity index shows a dissimilarity of almost 90 % regarding more abundant species. However, the diversity indices result in comparable values for Simpson index, Shannon–Wiener index, evenness, and Fisher's alpha index, with a general trend towards Site B sample being slightly more diverse.
The relationship between living and dead foraminifera shows a higher percentage of living individuals at Site B (14.89 %) compared to Site A (7.13 %).
The reasons for the significant differences in assemblage composition between both investigated sites are not entirely clear, as the level of metal pollution is not the only distinguishing factor. The differing grain size distributions undoubtedly play a major role as well (Romano et al., 2013). These variations in grain size are likely also attributable to anthropogenic influences and, indirectly, to pollution from industrial inputs.
Three out of the five dominant species found at Site A were completely absent at Site B, of which Ammoscalaria runiana and Ammobaculites exilis showed a potential affinity towards muddy–sandy sediment (Murray, 1991). This aligns well with the observation of an increase in fine sediment constituents within the same sample. Elphidium cf. advena is the most dominant species at Site A and is associated with muddy–sandy sediment (Murray, 1991) while also positively correlating with high metal concentrations of copper, iron, lead, and zinc (Romano et al. 2009). This might explain why the abundance of this species at Site A is vastly increased (almost 13-fold) compared to at Site B. Rarer species only found in Site A samples are Bolivina striatula, Buccella granulata, Nonionoides turgidus, and Reophax sp., which can be explained by their association with muddy sediment (Murray, 1991). Ammonia advena, Spirillina vivipara, and the two dominant species Eggerelloides scabrum and Ammoscalaria runiana are also partly exclusive to Site A but have previously been found in coarser sediment (Murray, 1991). Their presence at Site A cannot be explained by the analyzed parameters but could be connected to their preferred food sources; as shown by Hohenegger et al. (1989, 1993), the spatial distribution of foraminifera species is often patchy and can be influenced by the availability of certain food sources.
Additionally, benthic foraminifera show different tolerances to organic matter enrichment in the sediment. In the context of assessing the quality status of marine waters, benthic foraminifera are analyzed at species level, and indices such as the Ammonia-Elphidium Foraminiferal Index (Sen Gupta et al., 1996; Sen Gupta and Platon, 2006) or the Foram-AMBI index (Alve et al., 2016; Jorissen et al., 2018; Cavaliere et al., 2021) are employed. These indices primarily focus on organic matter enrichment. To date, no sensitivity index based on benthic foraminifera has been developed for metal pollution. As described by Lintner et al. (2021) and Bubl et al. (2024), various metals which occur simultaneously as pollutants (e.g., iron, lead, and zinc) affect benthic foraminifera differently (positively and negatively), which poses a challenge for potential metal-pollution-based foraminiferal indices.
A comparison of the abovementioned species identified in this study with the Foram-AMBI classifications for biomonitoring in the northeastern Atlantic and Arctic Sea (Alve et al., 2016), the Mediterranean Sea (Jorissen et al., 2018), and the Adriatic Sea (Žvab Rožič et al., 2022) reveals a mixed pattern. For instance, Elphidium advena is classified in ecological group II (indicating indifference to organic matter enrichment). The genus Ammoscalaria is only mentioned with a different species, also falling into category II. Nubeculina divaricata is categorized as group I (sensitive to organic matter enrichment), while Ammobaculites is not listed as a taxon. Despite being classified as a category III species (tolerant to excess organic matter enrichment), Eggerelloides scabrum can be found in comparable quantities in both Site A (5.35 %) and Site B (3.99 %). This could be explained by the aforementioned tendency towards coarser sediments of this particular species.
The less dominant species, which occur exclusively at Site A, are classified as follows: Bolivina striatula in group III, Buccella granulata in group I, Nonionoides turgidus in group V (first-order opportunistic species), Spirillina vivipara in group III, and species of the genus Reophax ranging between groups II and V. While Ammonia advena is not assessed, most species of the genus Ammonia are listed as either group II or group III.
Site B exhibits a broader spectrum of environmental preferences. All dominant species (Lobatula lobatula, Rosalina anomala, and Cibicides refulgens) are present at Site A as well but show notably higher abundances at Site B. This could be explained by their association with hard substrate (Murray, 1991) and, in the case of the most dominant species Lobatula lobatula, by a strong negative correlation with elevated metal concentrations of cadmium, chrome, copper, iron, lead, and zinc (Romano et al., 2009). Despite no evaluation for Rosalina anomala specifically, the general negative correlation with heavy metals that Romano et al. (2009) established for two other species of the genus Rosalina indicates the metals' inhibiting influence on this species.
The slightly rarer species Asterigerinata mamilla is exclusively found at Site B, although Romano et al. (2009) correlated it positively with arsenic. The absence of A. mamilla at Site A could be due to its strong negative correlation with iron, lead, and zinc and perhaps its positive correlation with gravel (Romano et al., 2009). Bulimina elongata, Lenticulina cultrata, and Melonis affinis are all exclusive to Site B, even though Murray (1991) associated all three genera with muddy sediment and Romano et al. (2009) demonstrated a positive correlation with chrome, magnesium, and manganese for Bulimina elongata. Other environmental factors might have prevented them from flourishing at Site A.
The genus Ammonia is almost completely absent from Site B: only one (dead) individual has been identified (Ammonia inflata, as described by Cimerman and Langer, 1991). Foraminifera of the genus Ammonia are usually ubiquitous in most marine coastal environments, regardless of environmental factors such as sediment and salinity, as they are very tolerant to environmental stressors and are often the dominant taxon in shallow-water environments (Walton and Sloan, 1990). Currently, it is not clear why this particular taxon is practically nonexistent at Site B.
All three dominant species at Site B have been described as group I taxa (Lobatula lobatula, Cibicides refulgens), or the vast majority of other species within the corresponding genus belong to group I (Rosalina anomala, which was not listed). Among the rarer taxa of site B, Asterigerinata mamilla is also placed in group I, while Bulimina elongata is assigned to group III. The taxon Lenticulina is not listed, and, for Melonis, only other species are included, allocated to group I and III.
This reinforces the viability of the Foram-AMBI index as an environmental monitoring tool. Despite not being fully applicable (large quantities of sampled taxa were not assigned to an ecological group yet), a preliminary evaluation of analyzed foraminifera via the Foram-AMBI index substantially corroborates the degree of pollution observed at the sampling sites.
Environmental aspects influencing foraminiferal assemblages proved to be complex and are not entirely understood; isolating and investigating a single pollutant results in inadequate and incomplete conclusions, as benthic communities are exposed to multiple contaminants (organic or inorganic) simultaneously (Boehnert et al., 2020). This is particularly true for coastal marine environments, which are prone to accumulate anthropogenic toxins but also suffer from rapidly fluctuating environmental conditions and subsequent natural stress (Boehnert et al., 2020). Food availability, another important aspect for marine ecosystems, may be positively influenced by the abundance of iron measured at the former steel mill dock; the comparatively larger amount of nutrients available for algae could consequently amplify their availability as a food source for benthic organisms, including foraminifera (Round, 1984; Loubere and Fariduddin, 2003; Bubl et al., 2024).
The strong discrepancy in iron content in the tests of the two specimens of Textularia bocki could be attributed to the increased prevalence of iron within the sediment of Site A, which functions as the building material for their tests. However, this discrepancy is not present in the EDX analysis of the agglutinated foraminifera Eggerelloides scabrum, implying a different underlying mechanism for the apparent sediment selectivity of Textularia bocki. Furthermore, despite the best efforts to choose individuals for EDX analysis with typical iron distribution patterns within their tests that are representative for an entire species of foraminifera, a range of variation among them is certainly given. For future research, a larger number of analyzed individuals for each species would establish a more reliable baseline.
4.3 Influence of iron on the foraminiferal metabolism
The positive correlation of certain species of foraminifera, particularly Elphidium cf. advena, with iron, zinc, and lead can be explained by observations from feeding experiments. Lintner et al. (2021) determined that individuals of the species Elphidium excavatum show severely limited metabolic functions as a reaction to highly elevated levels of zinc (144- and 1044-fold concentrations relative to seawater). However, exposure of the same species to 9.2-fold zinc concentrations increased food uptake, while not even 557-fold levels of lead had any significant negative impact on the foraminifera (Lintner et al., 2021). These results show once again that foraminifera react very sensitively to some elements, whereas other metals have little or no negative influence on their metabolism. Our study revealed that iron is still the most enriched metal at Site A and potentially also in the whole bay, as described several times in the past by Sprovieri et al. (2019). Especially in the region near Bagnoli, it is assumed that the high concentration of Fe and Cu is responsible for the fluctuations in the foraminiferal community (Bergamin et al., 2005). Bubl et al. (2024) investigated the influence of iron on the metabolic activity of Heterostegina depressa, a photosymbiont-bearing foraminifer. The incubation experiment revealed that even higher levels of iron (100-fold concentration relative to seawater) had a positive effect on metabolic functions of this foraminifer, while simultaneously reaffirming previous conclusions regarding zinc and lead (Bubl et al., 2024). The foraminifer Miliolinella subrotunda shows potential to incorporate Fe into tests (Romano et al., 2008). However, these metal incorporations are higher in deformed parts of the tests, which leads to the conclusion that Fe is harmful for this species in higher concentrations (Romano et al., 2018). Bergamin et al. (2005) found a negative correlation within the occurrence of this species and the metal concentration in the sediment, which results in the fact that M. subrotunda can be a potential bioindicator for metal pollution. In our study, we found 3 times more M. subrotunda at Site B compared to Site A. Since Site A has higher metal contamination, it can also be assumed that M. subrotunda has the potential to be used as a bioindicator for elevated heavy metal concentrations.
Ultimately, it is as yet uncertain whether these interactions with metals are universal among foraminifera or are the product of few well-adapted species. The obtained assemblage data imply that some species preferably inhabit this pollutant-impacted ecological niche. Based on research conducted by Frontalini et al. (2018), it can be assumed that intracellular defense mechanisms – and thus biochemical resilience against toxic metals – employed by foraminifera are independent of test material; both the hyaline Ammonia parkinsoniana and the miliolid Pseudotriloculina rotunda showed similar adaptions to toxic metal exposure, regardless of how the pollutants were introduced to their tests (extracellular biomineralization and intracellular biomineralization, respectively) (De Giudici et al., 2018; Frontalini et al., 2018). Subsequent studies could therefore investigate different genera and species of live foraminifera and their specific adaptations and reactions to metal pollution exposure. Ultimately, the long-term consequences of anthropogenic metal contamination on the local ecosystem need to be carefully contrasted with the short-term effects on individual benthic foraminifera.
All data used in this study are included in the article; species abundance data are available in Appendix A and at https://doi.org/10.5281/zenodo.15799673 (Plakolm and Nagy, 2025).
Conceptualization: ML. Formal analysis: LP, MN, ML. Funding acquisition: PH, ML. Investigation: LP, SB, MN, DG, MS, ML. Methodology: ML. Project administration: PH, ML. Resources: PH, KS, TH. Supervision: MN, PH, ML. Validation: LP, MN, PH. Visualization: LP. Writing (original draft): LP. Writing (review and editing): LP, SB, MN, PH, DG, KS, MS, TH, ML.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This article is part of the special issue “Advances and challenges in modern and benthic foraminifera research: a special issue dedicated to Professor John Murray”. It is not associated with a conference.
Open-access funding was provided by the University of Vienna. The authors would like to thank Christian Baal (Department of Palaeontology, University of Vienna) for his contributions to SEM imaging. The authors are also grateful for constructive criticism and feedback from two anonymous reviewers and the editor.
The research leading to these results received partial funding from the European Union's Horizon 2020 research and innovation program under grant no. 730984, ASSEMBLE Plus project (Foraminifera in Naples experiencing systematic toxicity; project code: 11247).
This paper was edited by Malcolm Hart and reviewed by two anonymous referees.
Albanese, S., De Vivo, B., Lima, A., Cicchella, D., Civitillo, D., and Cosenza, A.: Geochemical baselines and risk assessment of the Bagnoli brownfield site coastal sea sediments (Naples, Italy), J. Geochem. Explor., 105, 19–33, https://doi.org/10.1016/j.gexplo.2010.01.007, 2010.
Alve, E., Korsun, S., Schönfeld, J., Dijkstra, N., Golikova, E., Hess, S., Husum, K., and Panieri, G.: Foram-AMBI: A sensitivity index based on benthic foraminiferal faunas from North-East Atlantic and Arctic fjords, continental shelves and slopes, Mar. Micropaleontol., 122, 1–12, https://doi.org/10.1016/j.marmicro.2015.11.001, 2016.
Andersen, J. H., Harvey, T., Murray, C., Green, N., and Reker, J.: Contaminants in Europe's seas: Moving towards a clean, non-toxic marine environment, European Environment Agency, https://www.eea.europa.eu/publications/contaminants-in-europes-seas (last access: 3 December 2024), 2019.
Barwell, L. J., Isaac, N. J. B., and Kunin, W. E.: Measuring β-diversity with species abundance data, J. Anim. Ecol., 84, 1112–1122, https://doi.org/10.1111/1365-2656.12362, 2015.
Bergamin, L., Romano, E., Magno, M. C., Ausili, A., and Gabellini, M.: Pollution monitoring of Bagnoli Bay (Tyrrhenian Sea, Naples, Italy), a sedimentological, chemical and ecological approach, Aquat. Ecosyst. Health, 8, 293–302, https://doi.org/10.1080/14634980500220866, 2005.
Binczewska, A., Polovodova Asteman, I., and Farmer, E. J.: Foraminifers (Benthic), in: Encyclopedia of Marine Geosciences, edited by: Harff, J., Meschede, M., Petersen, S., and Thiede, J., Springer Netherlands, 251–255, https://doi.org/10.1007/978-94-007-6644-0_60-1, 2015.
Boehnert, S., Birkelund, A. R., Schmiedl, G., Kuhnert, H., Kuhn, G., Hass, H. C., and Hebbeln, D.: Test deformation and chemistry of foraminifera as response to anthropogenic toxic metal input, Mar. Pollut. Bull., 155, 111112, https://doi.org/10.1016/j.marpolbul.2020.111112, 2020.
Bouchet, V. M. P., Alve, E., Rygg, B., and Telford, R. J.: Benthic foraminifera provide a promising tool for ecological quality assessment of marine waters, Ecol. Indic., 23, 66–75, https://doi.org/10.1016/j.ecolind.2012.03.011, 2012.
Bradl, H. B.: Sources and Origins of Toxic Metals, in: Interface Science and Technology: Vol. 6. Toxic Metals in the Environment, edited by: Bradl, H. B., Elsevier, 1–27, ISBN 0120883813, 2005.
Bubl, M., Heinz, P., Wanek, W., Schagerl, M., Hofmann, T., and Lintner, M.: Impact of heavy metals (Cu, Fe, Pb, Zn) on carbon and nitrogen uptake of the diatom-bearing benthic foraminifera Heterostegina depressa, Heliyon, 10, e27229, https://doi.org/10.1016/j.heliyon.2024.e27229, 2024.
Carpenter, W. B.: XXVI. Researches on the foraminifera. Part II. On the genera Orbiculina, Alveolina, Cycloclypeus and Heterostegina, Philos. T. Roy. Soc. Lond., 146, 547–569, https://doi.org/10.1098/rstl.1856.0027, 1856.
Cavaliere, M., Barrenechea Angeles, I., Montresor, M., Bucci, C., Brocani, L., Balassi, E., Margiotta, F., Francescangeli, F., Bouchet, V. M. P., Pawlowski, J., and Frontalini, F.: Assessing the ecological quality status of the highly polluted Bagnoli area (Tyrrhenian Sea, Italy) using foraminiferal eDNA metabarcoding, Sci. Total Environ., 790, 147871, https://doi.org/10.1016/j.scitotenv.2021.147871, 2021.
Cimerman, F. and Langer, M. R.: Mediterranean Foraminifera, Slovenian Academy of Sciences and Arts, Swiss Academy of Natural Sciences, Scientific Research Center of the Slovenian Academy of Sciences and Arts, 1991.
Coccioni, R. and Marsili, A.: Monitoring in polluted transitional marine environments using foraminifera as bioindicators: a case study from the Venice Lagoon (Italy), in: Vol. 3, Dossier No. 3, Proceedings of the International Conference, 26–28 April 2004, Venice, IOC ICAM – Integrated Coastal Area Management, UNESCO, 250–256, 2005.
De Giudici, G., Meneghini, C., Medas, D., Buosi, C., Zuddas, P., Iadecola, A., Mathon, O., Cherchi, A., and Kuncser, A. C.: Coordination environment of Zn in foraminifera Elphidium aculeatum and Quinqueloculina seminula shells from a polluted site, Chem. Geol., 477, 100–111, https://doi.org/10.1016/j.chemgeo.2017.12.009, 2018.
El-Kahawy, R. M. and Mabrouk, M. S.: Benthic foraminifera as bioindicators for the toxic metals in the severely polluted Hurghada Bay, Red Sea coast, Egypt, Environ. Sci. Pollut. Res., 30, 70437–70457, https://doi.org/10.1007/s11356-023-27242-4, 2023.
Fajemila, O. T., Martínez-Colón, M., and Spezzaferri, S.: Spatial distribution of pollution levels and assessment of benthic foraminifera in Apapa-Badagry Creek, Nigeria, Mar. Pollut. Bull., 185, 114359, https://doi.org/10.1016/j.marpolbul.2022.114359, 2022.
Ferraro, L., Sprovieri, M., Alberico, I., Lirer, F., Prevedello, L., and Marsella, E.: Benthic foraminifera and toxic metals distribution: A case study from the Naples Harbour (Tyrrhenian Sea, Southern Italy), Environ. Pollut., 142, 274–287, https://doi.org/10.1016/j.envpol.2005.10.026, 2006.
Ferraro, L., Sammartino, S., Feo, M. L., Rumolo, P., Manta, D. S., Marsella, E., and Sprovieri, M.: Utility of benthic foraminifera for biomonitoring of contamination in marine sediments: A case study from the Naples harbour (Southern Italy), J. Environ. Monitor., 11, 1226, https://doi.org/10.1039/b819975b, 2009.
Frontalini, F. and Coccioni, R. : Benthic foraminifera for toxic metal pollution monitoring: A case study from the central Adriatic Sea coast of Italy, Estuar. Coast. Shelf Sci., 76, 404–417, https://doi.org/10.1016/j.ecss.2007.07.024, 2008.
Frontalini, F., Buosi, C., Da Pelo, S., Coccioni, R., Cherchi, A., and Bucci, C.: Benthic foraminifera as bio-indicators of trace element pollution in the heavily contaminated Santa Gilla lagoon (Cagliari, Italy), Mar. Pollut. Bull., 58, 858–877, https://doi.org/10.1016/j.marpolbul.2009.01.015, 2009.
Frontalini, F., Nardelli, M. P., Curzi, D., Martín-González, A., Sabbatini, A., Negri, A., Losada, M. T., Gobbi, P., Coccioni, R., and Bernhard, J. M.: Benthic foraminiferal ultrastructural alteration induced by toxic metals, Mar. Micropaleontol., 138, 83–89, https://doi.org/10.1016/j.marmicro.2017.10.009, 2018.
Gao, W., Qu, B., Yuan, H., Song, J., and Li, W.: Heavy metal mobility in contaminated sediments under seawater acidification, Mar. Pollut. Bull., 192, 115062, https://doi.org/10.1016/j.marpolbul.2023.115062, 2023.
Geslin, E., Stouff, V., Debenay, J.-P. and Lesourd, M.: Environmental Variation and Foraminiferal Test Abnormalities, in: Topics in Geobiology: Vol. 15. Environmental Micropaleontology, edited by: Martin, R. E., Kluwer Academic/Plenum Publishers, 191–215, https://doi.org/10.1007/978-1-4615-4167-7_10, 2000.
Goldstein, S. T.: Foraminifera: A biological overview, in: Modern Foraminifera, edited by: Sen Gupta, B. K., Kluwer Academic Publishers, 37–55, https://doi.org/10.1007/0-306-48104-9, 2003.
Hallock, P.: Symbiont-Bearing Foraminifera, in: Modern Foraminifera, edited by: Sen Gupta, B. K., Kluwer Academic Publishers, 123–139, https://doi.org/10.1007/0-306-48104-9, 2003.
Hammer, Ø., Harper, D. A. T. and Ryan, P. D.: PAST: Paleontological statistics software package for education and data analysis, Palaeontol. Electron., 4, 9, http://palaeo-electronica.org/2001_1/past/issue1_01.htm, 2001.
Hart, M. B., Stubbles, S. J., Smart, C. W., Fisher, J. K., Hoddinott, C., Marshall-Penn, I., and Yeo, A.: Foraminifera from the Fowey Estuary, Cornwall, Geosci. SW Engl., 13, 304–315, 2014.
Hendriks, H. C. M., van Prooijen, B. C., Aarninkhof, S. G. J., and Winterwerp, J. C.: How human activities affect the fine sediment distribution in the Dutch Coastal Zone seabed, Geomorphology, 367, 107314, https://doi.org/10.1016/j.geomorph.2020.107314, 2020.
Hohenegger, J., Piller, W., and Baal, C.: Reasons for Spatial Microdistributions of Foraminifers in an Intertidal Pool (Northern Adriatic Sea), Mar. Ecol., 10, 43–78, https://doi.org/10.1111/j.1439-0485.1989.tb00065.x, 1989.
Hohenegger, J., Piller, W. E., and Baal, C.: Horizontal and vertical spatial microdistribution of foraminifers in the shallow subtidal Gulf of Trieste, northern Adriatic Sea, J. Foramin. Res., 23, 79–101, https://doi.org/10.2113/gsjfr.23.2.79, 1993.
Jeong, H., Araújo, D. F., Knœry, J., Briant, N., and Ra, K.: Isotopic (Cu, Zn, and Pb) and elemental fingerprints of antifouling paints and their potential use for environmental forensic investigations, Environ. Pollut., 322, 121176, https://doi.org/10.1016/j.envpol.2023.121176, 2023.
Jorissen, F., Nardelli, M. P., Almogi-Labin, A., Barras, C., Bergamin, L., Bicchi, E., El Kateb, A., Ferraro, L., McGann, M., Morigi, C., Romano, E., Sabbatini, A., Schweizer, M., and Spezzaferri, S.: Developing Foram-AMBI for biomonitoring in the Mediterranean: Species assignments to ecological categories, Mar. Micropaleontol., 140, 33–45, https://doi.org/10.1016/j.marmicro.2017.12.006, 2018.
Kok, V. C., Winn, P. R., Hsieh, Y.-J., Chien, J.-W., Yang, J.-M., and Yeh, G.-P.: A Pilot Survey of Potentially Hazardous Trace Elements in the Aquatic Environment Near a Coastal Coal-Fired Power Plant in Taiwan, Environ. Health Insights, 13, https://doi.org/10.1177/1178630219862236, 2019.
Le Cadre, V. and Debenay, J.-P.: Morphological and cytological responses of Ammonia (foraminifera) to copper contamination: Implication for the use of foraminifera as bioindicators of pollution, Environ. Pollut., 143, 304–317, https://doi.org/10.1016/j.envpol.2005.11.033, 2006.
Lintner, M., Lintner, B., Wanek, W., Keul, N., von der Kammer, F., Hofmann, T., and Heinz, P.: Effects of toxic elements (Pb, Cu, Zn) on algal food uptake by Elphidium excavatum (Foraminifera), Heliyon, 7, e08427, https://doi.org/10.1016/j.heliyon.2021.e08427, 2021.
Loubere, P. and Fariduddin, M.: Benthic Foraminifera and the flux of organic carbon to the seabed, in: Modern Foraminifera, edited by: Sen Gupta, B. K., Kluwer Academic Publishers, 181–199, https://doi.org/10.1007/0-306-48104-9, 2003.
Murray, J. W.: Ecology and Palaeoecology of Benthic Foraminifera, Routledge, https://doi.org/10.4324/9781315846101, 1991.
Nardelli, M. P., Malferrari, D., Ferretti, A., Bartolini, A., Sabbatini, A., and Negri, A.: Zinc incorporation in the miliolid foraminifer Pseudotriloculina rotunda under laboratory conditions, Mar. Micropaleontol., 126, 42–49, https://doi.org/10.1016/j.marmicro.2016.06.001, 2016.
OpenStreetMaps contributors: Map of the research area in the Gulf of Naples, https://www.openstreetmap.org (last access: 8 May 2023), 2023.
Passaro, S., Gherardi, S., Romano, E., Ausili, A., Sesta, G., Pierfranceschi, G., Tamburrino, S., and Sprovieri, M.: Coupled geophysics and geochemistry to record recent coastal changes of contaminated sites of the Bagnoli industrial area, Southern Italy, Estuar. Coast. Shelf Sci., 246, 107036, https://doi.org/10.1016/j.ecss.2020.107036, 2020.
Pawlowski, J.: Foraminifera, in: Eukaryotic microbes, edited by: Schaechter, M., Elsevier/Academic Press, 291–309, ISBN 9780128103661, 2012.
Plakolm, L. and Nagy, M.: Assessment of anthropogenic metal pollution in Bagnoli (Gulf of Naples) through changes in foraminiferal assemblages and shell chemistry, Zenodo [data set], https://doi.org/10.5281/zenodo.15799674, 2025.
Romano, E., Ausili, A., Zharova, N., Celia Magno, M., Pavoni, B., and Gabellini, M.: Marine sediment contamination of an industrial site at Port of Bagnoli, Gulf of Naples, Southern Italy, Mar. Pollut. Bull., 49, 487–495, https://doi.org/10.1016/j.marpolbul.2004.03.014, 2004.
Romano, E., Bergamin, L., Finoia, M. G., Carboni, M. G., Ausili, A., and Gabellini, M.: Industrial pollution at Bagnoli (Naples, Italy): benthic foraminifera as a tool in integrated programs of environmental characterisation, Mar. Pollut. Bull., 56, 439-457, https://doi.org/10.1016/j.marpolbul.2007.11.003, 2008.
Romano, E., Bergamin, L., Ausili, A., Pierfranceschi, G., Maggi, C., Sesta, G., and Gabellini, M.: The impact of the Bagnoli industrial site (Naples, Italy) on sea-bottom environment. Chemical and textural features of sediments and the related response of benthic foraminifera, Mar. Pollut. Bull., 59, 245–256, https://doi.org/10.1016/j.marpolbul.2009.09.017, 2009.
Romano, E., Bergamin, L., Magno, M. C., and Ausili, A.: Sediment characterization of the highly impacted Augusta harbour (Sicily, Italy): modern benthic foraminifera in relation to grain-size and sediment geochemistry, Environ. Sci.-Proc. Imp., 15, 930–946, 2013.
Romano, E., Bergamin, L., Magno, M. C., Pierfranceschi, G., and Ausili, A.: Temporal changes of metal and trace element contamination in marine sediments due to a steel plant: The case study of Bagnoli (Naples, Italy), Appl. Geochem., 88, 85–94, 2018.
Round, F. E.: The ecology of algae, Cambridge University Press, ISBN 9780521269063, 1984.
Samir, A. M.: The response of benthic Foraminifera and Ostracods to various pollution sources: a study from two lagoons in Egypt, J. Foramin. Res., 30, 83–98, https://doi.org/10.2113/0300083, 2000.
Schmidt, S., Hathorne, E. C., Schönfeld, J., and Garbe-Schönberg, D.: Heavy metal uptake of nearshore benthic foraminifera during multi-metal culturing experiments, Biogeosciences, 19, 629–664, https://doi.org/10.5194/bg-19-629-2022, 2022.
Schönfeld, J., Alve, E., Geslin, E., Jorissen, F., Korsun, S., Spezzaferri, S., Abramovich, S., Almogi-Labin, A., Armynot du Chatelet, E., Barras C., Bergamin, L., Bicchi, E., Bouchet, V., Cearreta, A., Di Bella, L., Dijkstra, N., Trevisan Disaro, S., Ferraro, L., Frontalini, F., Gennari, G., Golikova, E., Haynert, K., Hess, S., Husum, K., Martins, V., McGann, M., Oron, S., Romano, E., Sousa, S. M., and Tsujimoto, A.: The FOBIMO (FOraminiferal BIo-MOnitoring) initiative – Towards a standardised protocol for soft-bottom benthic foraminiferal monitoring studies, Mar. Micropaleontol., 94–95, 1–13, https://doi.org/10.1016/j.marmicro.2012.06.001, 2012.
Sen Gupta, B. K. (Ed.): Introduction to modern Foraminifera, in: Modern Foraminifera, Kluwer Academic Publishers, 3–6, https://doi.org/10.1007/0-306-48104-9, 2003.
Sen Gupta, B. K. and Platon, E.: Tracking Past Sedimentary Records of Oxygen Depletion in Coastal Waters: Use of the Ammonia-Elphidium Foraminiferal Index, Special Issue No. 39, Proceedings of the 8th International Coastal Symposium (ICS 2004), Vol. III, J. Coast. Res., 1351–1355, http://www.jstor.org/stable/25742974, 2006.
Sen Gupta, B. K., Turner, R. E., and Rabalais, N. N.: Seasonal oxygen depletion in continental-shelf waters of Louisiana: Historical record of benthic foraminifers, Geology, 24, 227, https://doi.org/10.1130/0091-7613(1996)024<0227:sodics>2.3.co;2, 1996.
Sprovieri, M., Passaro, S., Ausili, A., Bergamin, L., Finoia, M. G., Gherardi, S., Molisso, F., Quinci, E. M., Sacchi, M., Sesta, G., Trincardi, F., and Romano, E.: Integrated approach of multiple environmental datasets for the assessment of sediment contamination in marine areas affected by long-lasting industrial activity: the case study of Bagnoli (southern Italy), J. Soils Sediments, 20, 1692–1705, https://doi.org/10.1007/s11368-019-02530-0, 2019.
Sreenivasulu, G., Jayaraju, N., Reddy, B. C. S. R., Prasad, T. L., Nagalakshmi, K., and Lakshmanna, B.: Foraminiferal research in coastal ecosystems of India during the past decade: A review, GeoRes. J., 13, 38–48, https://doi.org/10.1016/j.grj.2017.02.003, 2017.
Stephenson, C. M., Hallock, P., and Kelmo, F.: Foraminiferal assemblage indices: A comparison of sediment and reef rubble samples from Conch Reef, Florida, USA, Ecol. Indic., 48, 1–7, https://doi.org/10.1016/j.ecolind.2014.07.004, 2015.
Stubbles, S. J.: Responses of Recent benthic foraminifera to metal pollution in South West England estuaries: a study of impact and change, University of Plymouth, https://doi.org/10.24382/4300, 1999.
Walton, W. R. and Sloan, B. J.: The genus Ammonia Bruennich, 1772; its geographic distribution and morphologic variability, J. Foramin. Res., 20, 128–156, https://doi.org/10.2113/gsjfr.20.2.128, 1990.
Yanko, V., Ahmad, M., and Kaminski, M. A.: Morphological deformities of benthic foraminiferal tests in response to pollution by toxic metals: Implications for pollution monitoring, J. Foramin. Res., 28, 177–200, 1998.
Yanko, V., Arnold, A. J., and Parker, W. C.: Effects of marine pollution on benthic Foraminifera, in: Modern Foraminifera, edited by: Sen Gupta, B. K., Kluwer Academic Publishers, 217–235, https://doi.org/10.1007/0-306-48104-9, 2003.
Žvab Rožič, P., Vidović, J., Ćosović, V., Hlebec, A., Rožič, B., and Dolenec, M.: A Multiparametric Approach to Unravelling the Geoenvironmental Conditions in Sediments of Bay of Koper (NE Adriatic Sea): Indicators of Benthic Foraminifera and Geochemistry, Front. Mar. Sci., 9, 812622, https://doi.org/10.3389/fmars.2022.812622, 2022.