the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Comparative analysis of six common foraminiferal species of the genera Cassidulina, Paracassidulina, and Islandiella from the Arctic–North Atlantic domain
Anna J. Pieńkowski
Anne Jennings
Karen Luise Knudsen
Marit-Solveig Seidenkrantz
Morphologically similar benthic foraminiferal taxa can be difficult to separate. Aside from causing issues in taxonomy, incorrect identifications complicate our understanding of species-specific ecological preferences and result in flawed palaeoenvironmental reconstructions and geochemical results. Over the years, a number of studies have grouped together several key Arctic–North Atlantic species in various combinations, despite their distinct environmental preferences and/or stratigraphical differences, causing great confusion in the literature. These species include Cassidulina laevigata, Cassidulina neoteretis, Cassidulina teretis, Paracassidulina neocarinata, Islandiella helenae, and Islandiella norcrossi. Here, we provide for the first time a detailed comparison of these taxa. We present a compilation of the original species descriptions, along with clear, illustrated guidelines on how to separate these taxa to circumvent taxonomic confusion. We acknowledge that some features cannot easily be seen with a standard low-powered microscope, especially if specimens are not well preserved. In those cases, we recommend the following actions: (i) always strive to make a precise identification and at least differentiate between the three genera; (ii) where C. neoteretis and C. teretis cannot be separated, and where the stratigraphical context does not make the species identification obvious, specimens belonging to these taxa should be reported as C. teretis/C. neoteretis; and (iii) where specimens in a sample cannot be confidently assigned to a specific species of Islandiella or Cassidulina, specimens should be grouped as Islandiella spp. or Cassidulina spp., followed by naming the most dominant species in brackets. The improved identification of Cassidulina, Paracassidulina, and Islandiella specimens will ensure development of a better understanding of the ecological affinities of these key Arctic–North Atlantic taxa, consequently resulting in more accurate palaeoenvironmental reconstructions and geochemical data.
- Article
(15844 KB) - Full-text XML
-
Supplement
(823 KB) - BibTeX
- EndNote
A common problem in benthic foraminiferal studies is the persistent taxonomic confusion between morphologically similar taxa, even among species that are often abundant. A consequence of this confusion can be the grouping and misidentification of key foraminiferal species that have different environmental preferences or even stratigraphically different distributions (e.g. Sejrup and Guilbault, 1980; Mackensen et al., 1985; Polyak et al., 2002), resulting in the loss of important information when analysing foraminiferal assemblage composition. It may also cause mixing of taxa when picking specimens for geochemical analyses, leading to potentially flawed data, especially when considering vital effects. This problem is often exacerbated by poor access to well-illustrated and high-quality taxonomic resources (with clear scanning electron microscope (SEM), light microscope or line illustrations, and taxonomic descriptions), along with information on changes in synonyms over time or regional use of species names in disagreement with type descriptions (e.g. Scott et al., 2000). Such confusion and “grouping” of species can lead to inaccurate interpretations of both modern and past environments, which then enter the literature and further contribute towards the problem. Ideally, researchers lacking access to quality taxonomic resources would seek to examine type material available at museums or other institutions or visit experts for training; however, this is not always possible due to funding issues or lack of mobility and opportunity.
Morphologically similar genera of benthic foraminifera found in Arctic and North Atlantic environments include Cassidulina, Paracassidulina, and Islandiella, particularly the biserially enrolled, lenticular-shaped species Cassidulina neoteretis, Cassidulina teretis, Cassidulina laevigata, Paracassidulina neocarinata, Islandiella helenae, and Islandiella norcrossi (e.g. Hunt and Corliss, 1993; Scott et al., 2008; Hanslik and Hermelin, 2011; Skirbekk et al., 2016). These hyaline species all possess a biserially enrolled, biconvex, lenticular test with a generally sharp to keeled margin. Some of the species most commonly display an undulating margin, whereas in others the margin is mainly straight. When well preserved, these taxa all have clearly visible chambers (often similar in number) that reach towards the umbilical area with triangular or curved ends that may or may not meet at the umbilicus; however, this can be more difficult to identify in abraded, non-translucent specimens. The aperture always sits on one side; for Cassidulina it is a narrow apertural slit with an apertural plate, whereas for Islandiella the aperture is a broader triangular to elliptical opening on the margin of the final chamber (Table 1). In Paracassidulina it is a narrow slit just as in Cassidulina, but it is longer and has a very thin apertural plate looking more like a lip than an actual plate.
Table 1Key morphological characteristics of Cassidulina, Paracassidulina and Islandiella taxa discussed in this study.
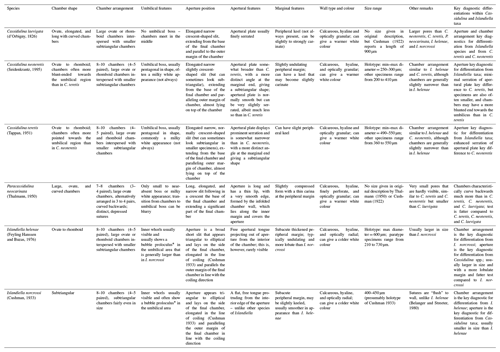
* Size of proloculus is dependent on type of sexual reproduction – sometimes it is so small in Islandiella taxa that it is difficult to see.
Despite their overall morphological similarities, the six species in question are reported to have very different environmental preferences. For example, C. neoteretis is found primarily in cool, stable water masses, typically in Atlantic-sourced subsurface waters in arctic and subarctic regions (Jennings and Helgadóttir, 1994; Seidenkrantz, 1995; Rytter et al., 2002), whereas C. laevigata is common in warmer, boreal (i.e. temperate) waters (Klitgaard-Kristensen et al., 2002; Jennings et al., 2004). C. teretis is believed to be extinct (Seidenkrantz, 1995; Lazar et al. 2016) and had a habitat extending into shallower waters than C. neoteretis (see Feyling-Hanssen et al., 1983; Feyling-Hanssen, 1980a; Seidenkrantz, 1995). The ecology of P. neocarinata is less well known, as this taxon is less frequently reported, likely due to its confusion with C. laevigata. However, P. neocarinata has been found in boreal to lusitanian (temperate to (sub)tropical) regions (e.g. Vilks and Rashid, 1976; Sen Gupta and Aharon, 1994). Confusing these four species would therefore result in very misleading palaeoenvironmental interpretations.
Additionally, taxonomic confusion or grouping of species in modern environments obscures our understanding of the ecological preferences of specific taxa, perhaps most prominently illustrated by the persistent grouping of I. helenae and I. norcrossi (e.g. Hald and Steinsund, 1996; Korsun and Hald, 1998; Polyak et al., 2002; Saher et al., 2012; Ovsepyan and Taldenkova, 2019). I. norcrossi is found in chilled Atlantic water (e.g. Arctic Intermediate Water) (Rytter et al., 2002; Jennings et al., 2004), whereas I. helenae can tolerate low temperatures and lower salinities and seems to be an indicator of sea ice margin conditions (Jennings and Helgadóttir, 1994; Seidenkrantz, 2013); thus, distinguishing these two species is particularly important.
Feyling-Hanssen and Buzas (1976) provided a detailed description and explanation of the differences between Islandiella and Cassidulina. Despite this, taxa from the genera Cassidulina and Islandiella continue to be either grouped into one species group or partly misidentified (e.g. Scott et al., 2008, Islandiella teretis; Cronin et al., 2019). Here we present an illustrated comparative study of these six morphologically comparable species C. laevigata, C. neoteretis, C. teretis, P. neocarinata, I. helenae, and I. norcrossi (using published and unpublished images) and highlight the distinguishing characteristics that allow them to be correctly identified at a species level. Our aim is to provide clear criteria and guidelines on how these species can be taxonomically separated. Importantly, we do not redescribe the species; instead, our study includes the original taxonomic descriptions published for each species, along with observations and remarks on morphology, supported by high-quality light microscope and SEM images; however, in a few cases we add to the original descriptions. For each species, we also add ecological remarks that rely on information from “modern” samples and reference selected key literature to aid future (palaeo)environmental studies, although we are not presenting a full exhaustive literature review. This paper brings together, for the first time, taxonomic and ecological resources for these key Arctic–Atlantic species that are often not easily accessible to researchers in the field. Although some species are found outside the Arctic and North Atlantic region, we focus the present review on this region alone.
We use both published and hitherto unpublished images to illustrate the distinguishing and key morphological features of the species discussed in this paper (Figs. 1–6). In addition to studying the literature, we include specific observations on foraminifera from surface sediment and core samples from sites within the North American Arctic and the North Atlantic region (Supplement Table S1). The classification and taxonomy in the present paper are based on Loeblich and Tappan (1987). Where possible, the original descriptions, which are not always openly available, are also included for the six species discussed. For original descriptions of the species, also see Ellis and Messina (1949 and update). It is beyond the scope of this paper to detail and describe ontogenetic variability in the taxa of interest, but Nomura (1983) illustrates how the morphology of the tests can change over the lifetime of the taxa, and researchers should be aware of how this may contribute to misidentification.
-
Suborder Rotaliina Delage and Hérouard, 1896
-
Superfamily Cassidulinacea d'Orbigny, 1839
-
Family Cassidulinidae d'Orbigny, 1839
-
Subfamily Cassidulininae d'Orbigny, 1839
-
Genus: Cassidulina d'Orbigny, 1826
-
Type species: Cassidulina laevigata d'Orbigny, 1826, p. 282, pl. 15, figs. 4, 5.
The original description from d'Orbigny (1826) is as follows: “Loges assemblées sur deux ou alternantes; ouverture vers le milieu de la loge” (as cited in Nørvang, 1958). This can be translated as “chambers assembled on two axes or alternating; aperture towards the middle (centre) part of the chamber”.
An emendation was made by Nørvang (1958):
Test lenticular to subglobular; chambers biserially arranged, planispirally coiled; wall perforate, granulate; aperture narrow tripartite, but one or two of the narrow branches are normally closed, except in the most primitive species; often with one or two plate-like lips, situated on the normally inward-bent border of the test wall, partially closing the aperture. Differential diagnosis: this genus differs from genera Ehrenbergina and Cassidulinoides by being completely coiled. It is distinguished from the genus Islandiella by the granular wall structure and the lack of an internal tooth.
-
Cassidulina laevigata d'Orbigny, 1826
-
Fig. 1a–i
-
1826 Cassidulina laevigata d'Orbigny: 282, pl. 15, figs. 4, 5.
-
1896 Cassidulina laevigata var. carinata Silvestri: 104, pl. 4, figs. 10a–c.
-
1945 Cassidulina laevigata d'Orbigny: Nørvang: 43, text-fig. 9.
-
1953 Cassidulina laevigata d'Orbigny: Phleger et al., 44, pl. 9, figs. 32, 37.
-
1958 Cassidulina laevigata d'Orbigny: Nørvang: 38, pl. 9, figs. 27–31.
-
1971 Cassidulina carinata Silvestri: Murray: 187, pl. 78, figs. 1–5.
-
1980 Cassidulina carinata Silvestri: Rodrigues et al.: 54, pl. 5, figs. 3, 6, 9.
-
1981 Cassidulina laevigata d'Orbigny: Sejrup et al.: 290, pl. 1, fig. 5.
-
2004 Cassidulina laevigata d'Orbigny: Jennings et al.: pl. 1, fig. 134.
Original taxonomic description
Unfortunately, no detailed description is given by d'Orbigny (1826); however, limited taxonomic remarks are made in the main text and in the caption of the relevant plate. We provide these details here and show the original line drawings in Fig. 1a. Nørvang (1958) offers a more detailed description that is also included here.
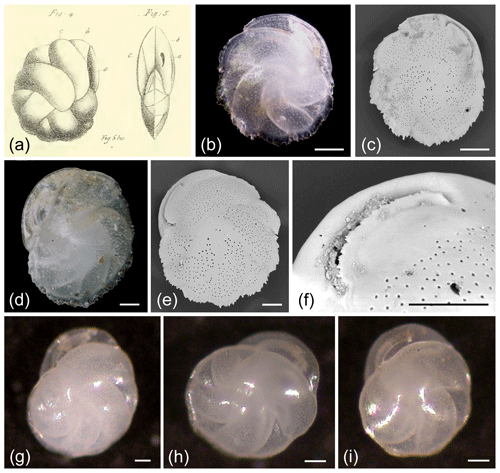
Figure 1Cassidulina laevigata. (a) Original line drawing by d'Orbigny (1826) showing the species in (left) profile and (right) apertural view. Note that the small dot at the base between the two illustrations refers to the natural size of the specimen in the original publication, which does not provide sizes or size ranges for C. laevigata. (b–f) C. laevigata carinate forms. (g–i) C. laevigata without a carinated keel and showing a more rounded form. (b) Composite depth-of-field light microscope image and (c) SEM micrograph of the same specimen. (d) Composite depth-of-field light microscope image and (e) SEM image of the same specimen. (f) SEM micrograph of aperture showing the broad apertural lip and carinate margin on the plate. (g–i) Light microscope images of C. laevigata specimens that show a smoother outline compared to (b)–(e). All scale bars denote 50 µm; see Supplement Table S1 for sample details.
The following description is taken from d'Orbigny (1826): “Loges non alternantes ou enfilées sur un seul axe. Côtés inégaux, l'un bombé et l'autre plat.” We translate this as follows: “Chambers not alternating or threaded on a single axis. Unequal sides, one convex and the other flat.” The following is from d'Orbigny's (1826) plate 15 caption – “Fig. 4 – Cassidulina laevigata grossie, vue de profil (par erreur citée pl. 6). Figure 5 Id. vue en face. a, l'ouverture virgulaire qui alterne dans l'accroissement des loges; b, la dernière loge venue, qui ne recouvre pas entièrement celle marquée c, qui est plus ancienne. Figure 5 bis. Id. Grandeur naturelle de la coquille.” This can be translated as follows: “Fig. 4 – Cassidulina laevigata magnified, profile view (plate 6 cited in error). Figure 5 – the same [species] in front (apertural) view: a, a virgulina opening alternating in the growth of the chambers; b, the last chamber which does not entirely cover that of the older chamber, marked c. Figure 5 is the same again. Natural size of the shell” (note that this is the small dot shown in the original).
Nørvang (1958) describes C. laevigata as follows:
Test lenticular; periphery carinated, or at least sharply angular; last whorl consisting of about four pairs of alternating chambers reaching in over the centre of the test, completely covering the chambers of the preceding whorl and leaving no umbilicus; chambers narrow, about three times as long as broad, distinctly curved; sutures distinct, more or less depressed, distinctly curved backwards from the centre of the test towards the periphery; aperture a long narrow slit formed by the areal branch only of the tripartite aperture – the basal branches being completely closed – starting the peripheral angle of the suture between the preceding chambers, traversing about two thirds of the long and narrow apertural face approximately conformable with the peripheral edge of the apertural face, thus in some specimens nearly touching the upper part of the apertural suture; wall along edge of aperture bent sharply inward, often with thin and narrow lips situated on the crest of the bent wall and partly closing the aperture.
Remarks
The original description of d'Orbigny (1826) lacks sufficient and clear detail (Fig. 1a), and this may have contributed to the extensive synonymization of this taxon, as emphasized by Nørvang (1945) and Mackensen and Hald (1988). Here we use Nørvang's (1958) description of Cassidulina laevigata. The primary diagnostic features of C. laevigata include the long, curved chambers that meet in the centre and result in the absence of an umbilical boss, along with the relatively large pores (Fig. 1) compared to C. neoteretis, C. teretis, and P. neocarinata (Fig. 5). The overall shape is compressed. C. laevigata exhibits a clear, elongated narrow crescent-shaped slit, which extends from the base of the final chamber and parallels the outer margin of the chamber. The aperture is partially closed by an apertural plate, which has a distinctly serrated (teeth-like) edge, although this is often only visible in well-preserved specimens (Seidenkrantz, 1995).
In previous studies, C. laevigata was often divided into what was thought to be two separate subspecies: Cassidulina laevigata laevigata (today usually called Cassidulina laevigata d'Orbigny, 1826) and C. laevigata carinata (also called Cassidulina carinata Silvestri, 1896; Murray, 1971; Rodrigues et al., 1980), with the main dividing characteristic being the absence (C. laevigata d'Orbigny, 1826; Fig. 1g–i) or presence (C. carinata Silvestri, 1896; Fig. 1b–e) of a peripheral keel. The keel can be slightly to distinctly carinate, with the carina often possessing a serrated to undulating edge. Often the carinate forms (C. carinata Silvestri, 1896) are rounder in side view, while the non-carinate forms (C. laevigata d'Orbigny, 1826) tend to be slightly longer than broad, sometimes having a weak kidney shape (Fig. 1g). However, although the endmembers of this group may be quite distinct, it is common to find transitional forms between carinate and non-carinate forms in some regions, and this can make distinguishing between these two subspecies difficult. Consequently, very few studies distinguish these forms. For example, Mackensen and Hald (1988) did not split their C. laevigata into the two subspecies C. laevigata laevigata d'Orbigny, 1826, and C. laevigata carinata due to these gradations. Murray (2003) also combined carinate and non-carinate forms of C. laevigata from the Hebridean Shelf in northwestern Scotland. As previous studies rarely mention which form(s) are present, making any distinction near impossible, here we treat the two types together under the name C. laevigata. It is currently unknown if any ecological differences exist between the carinate and non-carinate forms.
Environmental preferences
In general, C. laevigata is considered to be a shallow infaunal species that occurs in boreal (cool temperate) to lusitanian (sub-tropical) regions influenced by saline (Atlantic) water inflow (Jennings et al., 2004) and which prefers well-oxygenated conditions in mesotrophic and eutrophic environments (e.g. Mackensen and Hald, 1988; Klitgaard-Kristensen et al., 2002; Polovodova Asteman and Nordberg, 2013) with ample phytodetritus supply (Alve, 2010). In the Kattegat and Skagerrak (southern Scandinavia), C. laevigata inhabits deep basin (80–300 m) waters of relatively stable salinities in sandy to fine-grained sediments (Conradsen et al., 1994). This species appears off the northern coast of Norway (Hald and Steinsund, 1996), where its relative abundance has increased in the last few decades (Saher et al., 2012); it also inhabits the western Barents Sea, where the highest abundances are found in areas influenced by warm (>1 ∘C) Atlantic Water (Østby and Nagy, 1982). It is a common species on the southwestern Iceland shelf in Atlantic water of the Irminger Current (water temperatures 6.4–7 ∘C) and very sandy sediments at depths of 270–320 m (Jennings et al., 2004). The warm-water affinity of the species is also illustrated by its presence in the lusitanian (warm temperate) shelf waters of northwestern Scotland (e.g. Murray, 2003; Cage, 2005), the Mediterranean Sea (e.g. Milker and Schmiedl, 2012), and southwestern Africa (Schmiedl et al., 1997).
-
Cassidulina neoteretis Seidenkrantz, 1995
-
Figs. 2a, 2d–j, and 2o
-
1977 Cassidulina teretis Tappan (not Cassidulina teretis Tappan): Lagoe: 127, pl. 5, figs. 1.5, 16.
-
1980 Cassidulina teretis Tappan (not Cassidulina teretis Tappan): Rodrigues et al.: 59, pl. 2, figs. 1, 3, 5; pl. 5, figs. 1, 4, 7; pl. 6, figs. 7, 10.
-
1981 Cassidulina laevigata d'Orbigny (part; not Cassidulina laevigata d'Orbigny): Sejrup et al.: 290, pl. 1, fig. 5.
-
1987 Cassidulina teretis Tappan (not Cassidulina teretis Tappan): Mackensen: pl. 10, figs. e, f, k, 1.
-
1988 Cassidulina teretis Tappan (not Cassidulina teretis Tappan): Mackensen and Hald: 16–24, pl. 1, figs. 8–15.
-
1994 Cassidulina teretis Tappan (not Cassidulina teretis Tappan): Jennings and Helgadóttir: pl. 2, fig. 3.
-
1995 Cassidulina neoteretis n. sp. Seidenkrantz: 148, 151; pl.1, figs. 1–6 (holotype figs. 1a and 1b, paratypes figs. 2–6); pl. 2, figs. 1–14, pl. 3, figs. 1–8; pl. 5, figs. 1–3.
-
2004 Cassidulina neoteretis Seidenkrantz: Jennings et al.: pl. 1, fig. 14.
-
2008 Islandiella teretis (Tappan) (not Cassidulina teretis Tappan): Scott et al.: 248 (part), pl. 6, figs. 2–10, 13–14. (see Discussion Sect. 3.4: no such species as Islandiella teretis exists).
-
2011 Cassidulina laevigata d'Orbigny (not Cassidulina laevigata d'Orbigny): Schröder-Adams and van Rooyen, p. 323, figs. 4.14a, 4.14b.
-
2011 Cassidulina neoteretis Seidenkrantz: Schröder-Adams and van Rooyen: 323, fig. 4.15a, 4.15b.
-
2016 Cassidulina neoteretis Seidenkrantz: Lazar et al.: fig. 8.
-
2020 Cassidulina neoteretis Seidenkrantz: Jennings et al.: fig. 12, number 1.
The original diagnosis from Seidenkrantz (1995) is as follows: “Small, lenticular Cassidulina with 4 to 5 chamber pairs in the last whorl, a clear umbilical boss and a smooth triangular apertural plate.”
The original description from Seidenkrantz (1995) is as follows:
Test lenticular, biconvex with an acute, slightly undulating, peripheral margin. Umbilical boss of milky, semitranslucent shell material on each side. Eight to ten chambers (frequently 10) in the final whorl, biserially arranged in 4 to 5 alternating pairs, each chamber appearing large, rounded rhomboid to ovate and reaching to the umbilical boss on one side of the test, and small and subtriangular on the other side. Sutures distinct, thickened, but not limbate, slightly depressed and outlining the chamber. Wall calcareous, hyaline or opaque, and optically granular. Surface smooth with relatively small, rounded, pores evenly distributed on the chamber walls but with no pores on the umbilical boss or along the sutures. Aperture an elongate, narrow slit extending from the base of the final chamber in a crescent paralleling the outer margin of the chamber, reaching to the distance from the base of the chamber to the peripheral keel. A subtriangular apertural plate with a smooth edge, formed by the infolded chamber wall, lies along the inner margin and partly covers the aperture (a type H1 aperture with a slight tendency to a type H2 aperture, according to definition by Nomura, 1983) (pl. 3, figs. 1–8). A narrow, serrate ridge lies adjacent to the outer margin of the aperture (pl. 3, fig. 7). Holotype size: greatest diameter: 300 µm, least diameter: 250 µm, greatest thickness: 130 µm. Dimensions for 80 other specimens (average value in brackets): greatest diameter: 230–410 µm (300 µm), least diameter: 200–360 µm (260 µm), greatest thickness: 130–200 µm (150 µm).
Remarks
C. teretis and C. neoteretis exhibit very similar morphologies (Fig. 2) with a key diagnostic distinguishing feature being the prominent serration of the apertural plate in C. teretis (Fig. 2n), although a very slight serration may also sometimes be observed on the apertural plate of C. neoteretis (e.g. Fig. 2o; Seidenkrantz, 1995). In addition, the apertural plate of C. neoteretis often has a distinct elongated triangular shape, while that of C. teretis is most commonly crescent-shaped and serrated (Fig. 2); the angle of the aperture can normally be seen in light microscope images (if the aperture is preserved), as can the serration of the apertural plate in C. teretis (though we note that the C. teretis holotype is not well-preserved and that the serration is not readily apparent). Both Seidenkrantz (1995) and Lazar et al. (2016) use SEM micrographs to clearly show the differences in the degree of serration between the two species. However, whereas the angle of the aperture in C. neoteretis is clear, Lazar et al. (2016) highlight that the serration feature is extremely difficult, if not impossible, to observe using standard light microscopy in smaller (<125 µm) specimens that are often abundant in arctic environments. Furthermore, as shown by Seidenkrantz (1995), C. neoteretis is on average smaller in size than C. teretis (Table 1). Additionally, C. neoteretis often has a sharper periphery than C. teretis; this sharp margin can easily be seen using a light microscope. We observe in our samples that chambers of C. neoteretis typically, but not always, have a blunter end towards the umbilical region than C. teretis, which often has more pointy ends towards the umbilicus, and the umbilical region is commonly larger in C. teretis than in C. neoteretis (Fig. 2), although some specimens of C. teretis with an unusually small umbilical area on one side were noticed by Feyling-Hanssen et al. (1983) and Feyling-Hanssen (1990; both as Cassidulina cf. teretis).
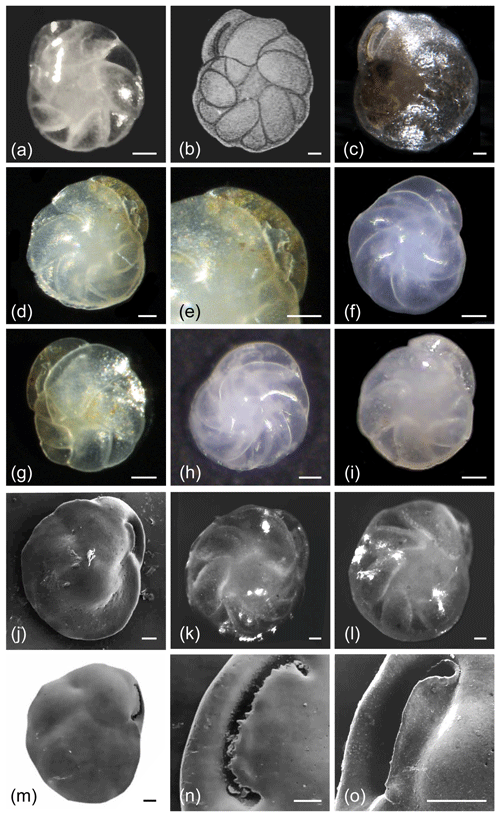
Figure 2Cassidulina neoteretis (a, d–j, o) and Cassidulina teretis (b–c, k–n). (a) Holotype image of C. neoteretis. (b) Original holotype illustration of C. teretis and (c) its re-image. (d–i) Light microscope images of C. neoteretis; the slightly carinate lip of the species is evident in (d)–(e) and (g), despite the flap of the aperture being slightly broken in both specimens. (k–l) Light microscope images of C. teretis. (j, m) SEM micrographs of C. neoteretis (j) and C. teretis (m). (n–o) Comparison between the apertural lips of (n) C. teretis vs. (o) C. neoteretis in SEM. All scale bars denote 50 µm, aside from (n)–(o) where they denote 25 µm. See Supplement Table S1 for sample details. (a, j–o) Reproduced from Seidenkrantz (1995) with the permission of the Journal of Micropalaeontology. (b) Reproduced with the permission of the Cushman Foundation for Foraminiferal Research. (c) Re-image from the Paleobiology Collections of the Smithsonian National Museum of Natural History (https://collections.nmnh.si.edu/search/paleo/, last access: 19 October 2020), made available under the Creative Commons CC0 1.0 licence.
The chamber arrangement of C. teretis and C. neoteretis makes them morphologically similar to I. helenae and I. norcrossi, and this can cause confusion in identification. We address this issue in the section on I. helenae and within the Discussion.
Environmental preferences
Today, C. neoteretis occurs in regions swept by Atlantic waters in arctic to subarctic and cold boreal regions. This shallow infaunal species typically dwells in fine-grained sediments in regions influenced by cool, modified Atlantic Water (with relatively stable salinities and temperatures) across the Nordic Seas (e.g. Mackensen and Hald, 1988; Gooday and Lambshead, 1989; Jennings and Helgadóttir, 1994; Rytter et al., 2002; Jennings et al., 2004; Knudsen et al., 2012), the Labrador Sea and Baffin Bay (Holocene records: Seidenkrantz et al., 2013; Hansen et al., 2020), and into the Arctic Ocean and its marginal seas (Wollenburg and Mackensen, 1998; Husum et al., 2015; Jennings et al., 2020). It is noteworthy that in the eastern Nordic Seas it is also found in Atlantic-sourced water at deeper sites with cooler and more stable conditions than usual for C. laevigata (cf. Mackensen and Hald, 1988). In the Arctic, it occurs in particularly high abundances and percentages in areas with a stratified water column, where chilled Atlantic waters are overlain by relatively fresh and cold polar waters (Jennings and Helgadottir, 1994; Jennings et al., 2004, 2020). In the North American Arctic, C. neoteretis appears only in areas influenced by subsurface Atlantic-origin water, such as M'Clure Strait in the Canadian Arctic Archipelago (cf. Vilks, 1969; Anna J. Pieńkowski, personal observation, 2020), the Beaufort Sea (Lagoe, 1980: as C. teretis), north of Ellesmere Island (Green, 1960: as C. teretis), and in northern Nares Strait (Jennings et al., 2020). It is abundant across the Arctic Ocean, particularly on the Chukchi Plateau (∼ 400–540 m depth) and on the Mendeleev Ridge and Lomonosov Ridge (both >1 km depth; Osterman et al., 1999: as C. teretis).
-
Cassidulina teretis Tappan, 1951
-
Figs. 2b–c and 2k–n
-
1951 Cassidulina teretis Tappan: 7–8, pl. 1, fig. 30.
-
1976a Cassidulina teretis Tappan: Feyling-Hanssen: 92, fig. 9, 4–5.
-
1976b Cassidulina teretis Tappan: Feyling-Hanssen: 354, pl. 1, fig. 13, pl. 2, figs. 20–21.
-
1980a Cassidulina teretis Tappan: Feyling-Hanssen: 173, pl. 4, figs. 10, 11, 15.
-
1983 Cassidulina cf. teretis Tappan: Feyling-Hanssen et al.: 105, pl. 1, figs. 6–9, 11–13.
-
1990 Cassidulina cf. teretis Tappan: Feyling-Hanssen: 22, pl. 4, figs. 10–14.
-
1995 Cassidulina teretis Tappan: Seidenkrantz: 151–153, pl. 1, figs. 12–14, pl. 2, figs. 15–18, pl. 4, figs. 1–5, pl. 5, fig. 4.
The original description from Tappan (1951) is as follows:
Test free, lenticular, with an umbilical boss on each side, composed of clear shell material; coiled and biserially arranged chambers alternating on the two sides of the peripheral keel, about eight to ten chambers visible along the periphery, chambers extending from the umbilical boss on one side, across the peripheral keel and about half-way to the umbilical boss of the opposite side, chambers appearing ovate in outline on the side where they reach the umbo, with the small subtriangular portion extending on the opposite side between the two adjacent ovate-appearing chambers of that side; sutures distinct and thickened but flush with the surface, gently curved; walls calcareous, with rather large perforations, surface smooth; aperture elongate, extending from the base of the final chamber in a crescent paralleling the anterior margin of the chamber, reaching nearly three-fourths the distance from the base of the chamber to the peripheral keel. Greatest diameter of holotype 0.55 mm; least diameter 0.49 mm; greatest thickness 0.23 mm. Other specimens are between 0.36 and 0.55 mm in greatest diameter.
Remarks
Although it is not mentioned in the original description by Tappan (1951), a normally crescent-shaped apertural plate with prominent serration (Seidenkrantz, 1995) is also noteworthy. C. teretis was first described from the Gubik Formation (Tappan 1951) from the late Pliocene or early Pleistocene. This species is believed to have become extinct in the Pleistocene, although the last occurrence datum is likely asynchronous over larger distances (Seidenkrantz, 1995; Lazar et al. 2016).
Environmental preferences
As no modern specimens of C. teretis have been identified (Seidenkrantz, 1995), its environmental preferences are based solely on palaeoenvironmental studies and evaluation of total foraminiferal assemblages in studies where we, based on available images or check of specimens, have ascertained that there is no doubt about the correct species identification: Feyling-Hanssen (1976a, b, 1980a, 1990) found C. teretis to be among the dominant species in Pliocene deposits from various sites on Baffin Island, northeastern Canada, together with, among others, Elphidium clavatum (Cushman, 1930), Haynesina orbiculare (Brady, 1881), Elphidium albiumbilicatum (Weiss, 1954), and Cassidulina inflata (Gudina, 1966), and from Kap København, northeastern Greenland (Feyling-Hanssen, 1990), and Lodin Elv, eastern Greenland (Feyling-Hanssen et al. 1983), together with, among others, E. clavatum, E. albiumbilicatum, and Cibicides grossus. It is also found in assemblages dominated by E. clavatum and C. grossus at Cape Chelyuskin in northern Russia (Möller et al., 2008). All of these above studies indicate an (inner) shelf environment. However, C. teretis has also been identified from the Pleistocene of the Arctic Ocean (Lazar et al., 2016), as well as from Pliocene–Pleistocene deposits in deeper-water conditions on the Vøring Plateau (Jansen et al. 1990) and in the North Sea region (Knudsen and Ásbjörnadóttir, 1991; Seidenkrantz, 1992), where it may extend stratigraphically to the Miocene (Jansen et al., 1990; Laursen et al., 1992).
-
Genus: Paracassidulina Nomura, 1983
-
Type species: Globocassidulina nipponensis Eade, 1969, p. 65.
The description from Nomura (1983) is as follows:
Test free, compressed circular to subauriculate in side view; periphery narrowly rounded; umbilicus commonly closed; chambers biserially arranged and enrolled as in Cassidulina, sometimes uncoiling in later, but continuing biserial development, increasing in size as added and overlapped at periphery; sutures commonly flush with surface, sometimes distinctly depressed; wall calcareous, finely perforate, optically granular in texture; surface smooth, polished to dimly reflected, with or without irregularly distributed grooves; aperture an interiomarginal, long and narrow slit, paralleling to periphery of preceding chamber.
-
Paracassidulina neocarinata (Thalmann, 1950)
-
Fig. 3a–f
-
1884 Cassidulina laevigata d'Orbigny (part; not Cassidulina laevigata d'Orbigny): Brady: 428, pl. 54, fig. 2 (not fig. 3)
-
1922 Cassidulina laevigata d'Orbigny var. carinata new variety Cushman: 124, pl. 25, figs. 6–7 (not Cassidulina laevigata d'Orbigny var. carinata Silvestri, 1896).
-
1950 Cassidulina neocarinata new name – Thalmann: pts 3–4, p. 44.
-
1951 Cassidulina laevigata d'Orbigny, var. carinata Cushman: Phleger: 27, pl. 14, fig. 7a, b.
-
1954 Cassidulina neocarinata Thalmann: Parker: 536, pl. 11, fig. 3.
-
1980 Cassidulina neocarinata Thalmann: Rodrigues et al.: 58, pl. 5, figs. 2, 5, 8; pl. 6, figs. 3, 4.
-
1994 Cassidulina laevigata d'Orbigny (part; not Cassidulina laevigata d'Orbigny): Jones: 60, pl. 54, fig. 2 (not fig. 3)
-
1995 Paracassidulina neocarinata (Thalmann): Seidenkrantz: pl. 1, figs. 10–11.
-
2015 Paracassidulina neocarinata (Thalmann): Poag: 110–111, pl. 20, fig. 4a, b.
Description
Thalmann (1950) refers to the original description of Cushman (1922): “Test differing from the typical in the thinner, more compressed test, with a very distinct thin carina, forming the periphery of the test.” Due to the very limited existing detail, we provide a short description as follows: test lenticular, biconvex with a thin carina at the peripheral margin. Very small umbilical boss of milky, semitranslucent shell material on each side. Seven to eight chambers in the final whorl, biserially and alternately arranged in 3 to 4 pairs, thus with only 3-4 chambers clearly seen on each side. Each chamber appearing large, ovate, curved backwards, reaching to the umbilical boss on one side of the test, and very small, subtriangular on the other side. Sutures distinct, depressed, increasingly depressed towards the periphery, and outlining the chamber. Wall calcareous, hyaline or opaque and optically granular. Surface smooth with very small pores evenly distributed on the chamber wall. Aperture a long, elongate, narrow slit following in a crescent the base of the final chamber and extending a significant part of the final chamber. A thin lip, with a very smooth edge, formed by the infolded chamber wall, lies along the inner margin and covers the aperture.
Remarks
P. neocarinata shows some affinity to C. teretis, C. neoteretis and C. laevigata, but it is very distinct in having fewer, more curved, ovate chambers; a smooth, thin lip; and a tendency to have a greater thickness at the umbilicus compared to the diameter than the other three species. It has much smaller pores than C. laevigata and a smaller (to near absent) umbilical boss compared to C. teretis and C. neoteretis.
Environmental preferences
The species is described in low to moderate numbers in continental shelf and slope environments from the western side of the Atlantic Ocean at water depths of 40–1000 m, most commonly from 100 to 700 m, ranging from the Gulf of Mexico to the coast of Halifax (Cushman, 1922; Phleger, 1951; Phleger and Parker, 1951: all as C. laevigata var. carinata Cushman; Parker, 1954; Vilks and Rashid, 1976: as C. laevigata; Poag, 2015). It is common in the Gulf of Saint Lawrence (Tiffany Audet, Canada, unpublished data). In addition, Sen Gupta and Aharon (1994) found P. neocarinata dominating the foraminiferal assemblages at bathyal hydrocarbon vents in the Gulf of Mexico. Platon (2001) found no such relationship but concluded that it is common to abundant on the continental slope of the Gulf of Mexico, living in the upper 3 cm below the sediment–water interface. In fossil sediments, Tichenor (2013) indicated a link to hypoxic conditions in the Gulf of Mexico. Due to potential taxonomical confusion in the literature with C. laevigata, the environmental range of P. neocarinata is still somewhat uncertain, but none of the authors of this paper have yet seen the species in recent assemblages from the eastern side of the Atlantic, although well-preserved specimens are found in Holocene sediments at just over 2000 m water depth south of Iceland (Christiansen, 2018).
-
Genus: Islandiella Nørvang, 1958
-
Type species: Cassidulina islandica Nørvang, 1945, p. 41, 42, fig. 7.
The description from Nørvang (1958) is as follows:
Test lenticular to subglobular; chambers biserially arranged, planispirally coiled; wall perforate, radiate fibrous; aperture large triangular to slit-like, basal but extending up in the apertural face; with an internal tooth extending back from the posterior edge of the aperture to the anterior corner of the foramen of the preceding chamber with a free tongue projecting out of and partly closing the aperture.
Nørvang (1958) also adds a differential diagnosis: “This genus can easily be confused with the genus Cassidulina which it resembles in the arrangement of the chambers and in general appearance. It differs from the latter in the presence of an internal tooth and in the radiate texture of the wall.”
-
Islandiella helenae Feyling-Hanssen and Buzas, 1976
-
Fig. 4a–d
-
1953 Cassidulina teretis Tappan (not Cassidulina teretis Tappan): Loeblich and Tappan: 121, pl. 24, figs. 3–4.
-
1966 Cassidulina teretis Tappan (not Cassidulina teretis Tappan): Gudina, p. 62, pl. 5, fig. 9, pl. 6, fig. 1.
-
1969 Cassidulina teretis (Tappan) (not Cassidulina teretis Tappan): Gudina, p. 47, pl. 15, fig. 9.
-
1971 Islandiella teretis (Tappan) (not Cassidulina teretis Tappan): Feyling-Hanssen et al.: 249, pl. 8, figs. 3–6; pl. 18, fig. 13.
-
1976 Islandiella helenae Feyling-Hanssen and Buzas: 155–157, figs. 1–4
-
1976a Islandiella helenae Feyling-Hanssen and Buzas: Feyling-Hanssen: 93, fig. 9. 9–10.
-
1980b Islandiella helenae Feyling-Hanssen and Buzas: Feyling-Hanssen: 272–274, pl. 1, figs. 14–16.
-
1994 Islandiella helenae Feyling-Hanssen and Buzas: Jennings and Helgadottir: pl. 2, fig. 4.
-
2008 Islandiella teretis (Tappan) (not Cassidulina teretis Tappan): Scott et al.: 248 (part), pl. 4, figs. 9, 9a; pl. 6, fig. 1. (see also Discussion below: no such species as Islandiella teretis exists).
-
2019 Islandiella norcrossi (Cushman) (not Islandiella norcrossi Cushman): Ovsepyan and Taldenkova, pl. 1, figs. 1–11, 13–15; pl. 2, figs. 1–8.
The original diagnosis from Feyling-Hanssen and Buzas (1976) is as follows: “A translucent to hyaline Islandiella with subacute periphery and 5 pairs of broad chambers in the final whorl.”
The original description from Feyling-Hanssen and Buzas (1976) is as follows (partly from Loeblich and Tappan, 1953, p. 121):
Test lenticular, biconvex with a subacutely thickened periphery margin, evolutely coiled so that previous whorls are seen through the thick, clear shell material of the umbilical region; chambers 8–10 in the final whorl, biserially and alternatively arranged in 4 to 5 pairs, each chamber appearing large, rounded rhomboid to ovate on one side of the test and small, subtriangular on the others; sutures distinct, thickened, flush with the surface, outlining the chambers; wall calcareous, perforate, translucent to hyaline, very distinctly radial when viewed in polarized light, surface smooth, glistening; aperture a broad, short slit paralleling the periphery, and with a free apertural tongue projecting out of it. Greatest diameter of holotype of figs. 2–4, 0.60 mm, thickness 0.29 mm. Greatest diameter of paratype of fig. 1, 0.60 mm, thickness 0.23 mm (Loeblich and Tappan, 1953, pl. 24, fig. 3). Other paratypes range from 0.21 to 0.73 mm in diameter (Loeblich and Tappan, 1953, p. 121).
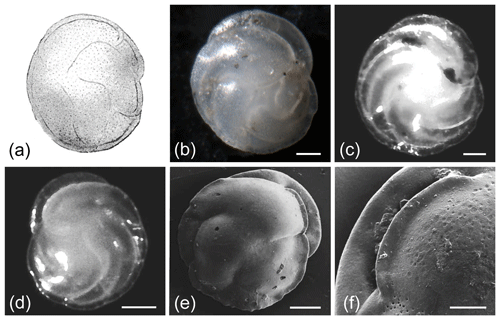
Figure 3Paracassidulina neocarinata. (a) Original holotype illustration and (b) an image of a syntype. Note that no information about size or size ranges is provided in the original description by Cushman (1922). (c–d) Light microscope images of P. neocarinata. (e–f) SEM micrographs of (e) the species, including (f) a detailed view of the aperture. All scale bars denote 50 µm, except for (f) where it denotes 25 µm. Sample details are given in Supplement Table S1. The original holotype illustration (a) is reproduced by permission from the Smithsonian Libraries; the syntype image (b) is from the Paleobiology Collections of the Smithsonian National Museum of Natural History (https://collections.nmnh.si.edu/search/paleo/, last access: 19 October 2020) and made available under the Creative Commons CC0 1.0 licence. (c–f) Reproduced from Seidenkrantz (1995), by permission from the Journal of Micropalaeontology.
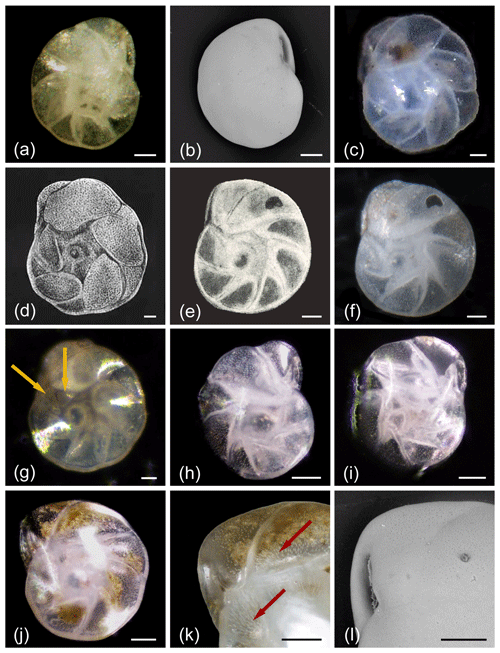
Figure 4Islandiella helenae and Islandiella norcrossi. (a, c) Light microscope images and (b) SEM images of I. helenae; note how easily these specimens may be mistaken for C. neoteretis if the apertural structure is overlooked. (d) Original holotype illustration of I. helenae. (e) Original holotype illustration of I. norcrossi and (f) its re-image. (g–k) Light microscope images and (l) SEM images of I. norcrossi. Notice the differences in proloculus size due to reproduction causing (h) megalospheric or (i) microspheric forms. (k) Close-up of pore tubules (shown by arrows) and the aperture of I. norcrossi in light microscopy. (l) Close-up of the aperture of I. norcrossi in SEM. (g) Light microscope image of I. norcrossi, showing a specimen that may potentially be misidentified as I. helenae were it not for the early triangular chambers (shown by arrows), which categorize it as an I. norcrossi All scale bars denote 50 µm aside from (k–l) where they show 25 µm. See Supplement Table S1 for sample details. The original holotype illustrations (d, e) are reproduced by permission from the Smithsonian Libraries. The re-image of the I. norcrossi holotype (f) is from the Paleobiology Collections of the Smithsonian National Museum of Natural History (https://collections.nmnh.si.edu/search/paleo/, last access: 3 March 2021) and made available under the Creative Commons CC0 1.0 licence.
Remarks
Similarities in morphological features between I. helenae and I. norcrossi (Fig. 4), particularly the “bubble” proloculus and gradations in the ideal chamber arrangements have led to difficulty in separating the species. Combined with the lack of a clear understanding of the differences in the ecological preferences of these species, this has often led to grouping of the two species in assemblage analyses and geochemical analyses (e.g. Korsun and Polyak, 1989; Hunt and Corliss, 1993; Hald and Steinsund, 1996; Korsun and Hald, 1998; Polyak et al., 2002; Rytter et al., 2002; Kristjansdóttir et al., 2007; Saher et al., 2012; Skirbekk et al., 2016). Failure to separate the two species has further obscured the understanding of species-specific environmental preferences. However complicated it may seem, these two taxa are readily distinguished by their chamber arrangements (Table 1, Figs. 4–5, 6). The chambers of I. norcrossi are triangular shaped and relatively even in size, whereas the chambers of I. helenae alternate in size between large rhomboid and ovate chambers interspersed with small subtriangular chambers. In fact, it is this alternating chamber arrangement that causes confusion between I. helenae and C. neoteretis but which allows I. norcrossi to be readily distinguished from I. helenae. A caveat worth mentioning is that an intergradation may appear between I. helenae and I. norcrossi. We have inspected a number of specimens of varying sizes from samples off northern Iceland (MD992272 and MD992275 both from ∼ 440 m water depth) and found that chambers may become increasingly irregular and rhomboid in larger specimens of I. norcrossi (e.g. Fig. 4g), and this can lead to misidentification as I. helenae. In this case it is important to evaluate the early chambers (i.e. the first two or three chambers in the last whorl), which will remain triangular in I. norcrossi. Additionally, the margins of the test are typically more lobate in I. helenae compared to I. norcrossi. Despite the distinct differences between well-formed specimens, there is a continued precedence for grouping these two taxa, which hinders ecological distribution studies (e.g. Hunt and Corliss, 1993; Steinsund, 1994; Korsun et al., 1995; Rytter et al., 2002; Scott et al., 2008; Polyak et al., 2013; Skirbekk et al., 2016).
Confusion also exists between I. helenae and C. neoteretis; however, Fig. 5 shows that despite the similarities in chamber arrangement, these two species can be quite easily and confidently taxonomically separated by careful examination of the aperture (Table 1). Furthermore, the chambers are broader in I. helenae than in C. neoteretis, and in well-preserved specimens of I. helenae and I. norcrossi, the fine perforations with distinct pore tubules are clearly visible, whereas these are absent in C. neoteretis. See the Discussion for more information.
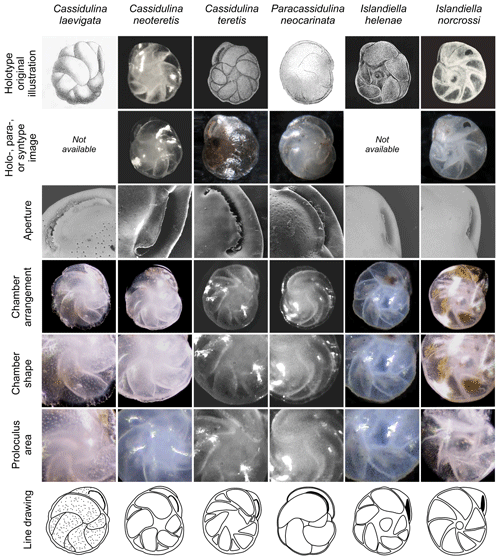
Figure 5Annotated comparison of Cassidulina laevigata, Cassidulina neoteretis, Cassidulina teretis, Paracassidulina neocarinata, Islandiella helenae, and Islandiella norcrossi, including original illustrations of the holotypes and holo-, para-, or syntype re-images where available. Key features for separating these morphologically similar species are highlighted. Holotype original illustrations are reproduced by permission from the Journal of Micropalaeontology (C. neoteretis), the Cushman Foundation for Foraminiferal Research (C. teretis), and the Smithsonian Libraries (P. neocarinata, I. helenae, I. norcrossi). Re-images for C. teretis (holotype), P. neocarinata (syntype), and I. norcrossi (holotype) are from the Paleobiology Collections of the Smithsonian National Museum of Natural History (https://collections.nmnh.si.edu/search/paleo/, last access: 19 October 2020) and are made available under the Creative Commons CC0 1.0 licence. A paratype image is provided for C. neoteretis from the material used in Seidenkrantz (1995). Line drawings in the bottom panel are based on original illustrations of holotypes and specimens examined for the present study. For sample details refer to Supplement Tables S1 and S2.
Environmental preferences
The frequent grouping of I. helenae and I. norcrossi has obscured their individual environmental preferences, in particular for I. helenae. This is likely due to I. helenae being observed less often than I. norcrossi (either living or dead); however, this species is often discussed as a group under I. norcrossi despite I. helenae sometimes being the dominant form (e.g. Korsun and Hald, 2000). I. helenae seems to bloom in sea ice marginal regions, likely due to the biological productivity in these environments (Jennings and Helgadóttir, 1994; Seidenkrantz 2013). In their assessment of foraminifera in the Kara Sea, Korsun and Hald (1998) used a category of “I. norcrossi s.l.” for specimens they considered transitional in morphology between the two taxa and show maximum abundances in glacial–distal settings, but where they could separate out I. helenae, they suggest that I. helenae prefers environments farther away from the glacier front. In the Barents Sea, Steinsund (1994) described I. helenae (in his section on I. norcrossi) from the relatively shallow and environmentally unstable areas of the Barents Sea. I. helenae is frequent on the relatively shallow shelf of the Canadian Arctic Archipelago (e.g. Vilks, 1989). In the modern Labrador Sea, I. helenae is particularly abundant on the inner shelf, where salinities are relatively low (∼ 34), along the path of the Labrador Current (Vilks et al., 1982; Mudie et al., 1984), in stark contrast to I. norcrossi, which is very rare in that area (Vilks et al., 1982). Jennings and Helgadóttir (1994) found living I. helenae on the inner southeastern Greenland shelf off Kangerlussuaq Fjord (site 3; 550 m water depth, 1.4 ∘C and 34.8 salinity) and low (live) percentages in outer Mikis Fjord (Site 11; 244 m water depth, −0.75 ∘C and a salinity of 33.8) and on the inner shelf (Site 16; 100 m water depth, no temperature and salinity data available). At the relatively shallow sites 11 and 16, I. helenae is between 6.7 % and 16.7 % of the >125 µm fraction and has a living component. I. norcrossi is uncommon (2 % or more commonly <1%) and not found living in any sample. I. helenae is associated with the sea ice edge in this area, where the sea ice edge moves through the area from winter to summer (Jennings and Helgadóttir, 1994). Furthermore, Lloyd (2006) reports ∼ 3 %–7 % of I. helenae in samples from Disko Bugt, western Greenland, where it is most common in assemblages otherwise dominated by agglutinated foraminifera, but also with the presence of C. neoteretis. This observation is consistent with its association with glacier ice-distal environments in eastern Novaya Zemlya (Korsun and Hald, 1998). Ovsepyan and Taldenkova (2019), in a study from the Laptev Sea, do not distinguish between I. helenae and I. norcrossi, naming them I. norcrossi, but as all specimens depicted in the species plates belong to I. helenae, we assume that the majority of their specimens are in fact I. helenae. They find a link to normal marine salinity and cold marine conditions on the outer shelf and continental slope, as well as to the high productivity in the seasonal sea ice marginal zone. In conclusion, the above findings suggest that I. helenae tolerates cold temperatures and a range of salinities from ∼ 33 to 34.5 in polar waters, also blooming in association with pulsed productivity at sea ice margins.
It is also noteworthy that whilst I. norcrossi may be found in large numbers in palaeo-records (e.g. Jennings et al., 2011; Hanssen et al., 2020), I. helenae is rarely common. When these Islandiella species are separated in palaeo-assemblages, their distributions show that they have distinct ecological preferences (e.g. Perner et al., 2013, 2015).
-
Islandiella norcrossi Cushman, 1933
-
Fig. 4e–l
-
1933 Cassidulina norcrossi Cushman: 7, pl. 2 fig. 7.
-
1953 Cassidulina norcrossi Cushman: Loeblich and Tappan: p. 120, pl. 24, fig 2.
-
1958 Islandiella norcrossi (Cushman): Nørvang: 32, pl. 7, figs. 8–11 (not figs. 12, 13); pl. 8, fig. 14.
-
1966 Planocassidulina norcrossi (Cushman): Gudina: 138, pl. 6, figs. 2, 3.
-
1971 Islandiella norcrossi (Cushman): Feyling-Hanssen et al.: 248, pl. 8, figs. 1–2.
-
1994 Islandiella norcrossi (Cushman): Jennings and Helgadottir: l. 2, fig. 5.
-
2004 Islandiella norcrossi (Cushman): Jennings et al.: pl. 1, fig. 15.
The original diagnosis from Cushman (1933) is as follows:
Test biconvex, periphery subacute or even slightly keeled; chambers distinct, generally triangular in side view, those of each set reaching nearly to the umbilicus on both sides; wall smooth, very finely perforate, clear and almost transparent; aperture elongate, in general in the line of coiling. Diameter, 0.40–0.45 mm; thickness, 0.15–0.18 mm.
Remarks
I. norcrossi exhibits visible inner whorls, with earlier chambers often giving a seemingly sawtooth pattern surrounding the umbilical region (Fig. 4e–j). It also frequently shows a small bubble proloculus in the umbilical area. This proloculus feature is often used to attribute specimens to I. norcrossi. However, the size of the proloculus differs in the microspheric and the macrospheric generation, sometimes being so small that it is hardly visible (observed in our own samples; Fig. 4i). However, a distinct and normally even larger bubble is also seen in I. helenae, making this a less distinct characteristic for I. norcrossi, although it may be used to separate I. helenae and I. norcrossi from Cassidulina. Yet, if the bubble is small or almost lacking, specimens may be mistakenly assigned to C. neoteretis. For this reason, examination of the chamber size and shape and the aperture is essential. I. norcrossi has a flat, free tongue protruding into its triangular aperture from the interior edge of the aperture (Fig. 4l), while I. helenae possesses a curled free tongue protruding from the interior of the final chamber. The free tongue of I. norcrossi looks rather similar to that of Cassidulina (cf. Rodrigues et al., 1980), although this feature may be difficult to observe in light microscopy. In this context, it is also important to note that there is a distinct difference in the microstructure of the two genera: Cassidulina being granular and Islandiella radial in microstructure. In well-preserved specimens, the fine perforations with distinct pore tubules in I. norcrossi are clearly visible (Fig. 4k).
Environmental preferences
I. norcrossi dwells in arctic to subarctic shelf and upper slope environments and has been reported down to ca. 1200 m water depth (Belanger and Streeter, 1980; Osterman et al., 1999). In the Norwegian-Greenland Sea, it is prominent down to 800 m depth, and its abundance declines in deeper waters (Belanger and Streeter, 1980). I. norcrossi is a common species on the North Iceland shelf east of 20∘ W in the cross-shelf troughs (water depths 300–500 m) that lead to the deeper waters of the Iceland Sea (Rytter et al., 2002; Jennings et al., 2004). Osterman et al. (1999) found I. norcrossi at a few sites on the Chukchi Shelf and slope. Hald and Korsun (1997) and Korsun and Hald (1998) encountered I. norcrossi in distal glaciomarine settings in the fjords of Svalbard and Novaya Zemlya, where it was reported to contribute towards 20 % of the assemblages. They suggested that I. norcrossi shows maximum abundances in the outer basins characterized by the inflow of Atlantic-sourced water, indicating a preference for relatively warmer diluted Atlantic waters; however, Korsun and Hald (1998) grouped I. helenae and I. norcrossi, so these reports may reflect a combination of the two species, thus obscuring the ecology of either species. Steinsund (1994) described typical forms of I. norcrossi from the deeper areas of the Barents Sea with stable water mass conditions, but also finds I. norcrossi in shallower, more environmentally unstable parts of the area. I. norcrossi is found at temperatures between −1 and +1 ∘C and is associated with relatively high and stable bottom-water salinity (Mudie et al., 1984; Steinsund et al., 1994; Korsun and Hald, 1998; Rytter et al., 2002; Jennings et al., 2004), which is in fact in accordance with Atlantic water blended with polar water (called chilled Atlantic Water or Arctic Intermediate Water depending on region). It occurs as up to 18 % of the assemblages in the upper Arctic Intermediate Water and Norwegian Sea Deep Water of Eyjafjardarall Trough and Hunaflóadjúp, North Iceland shelf, at water depths between 350 and 500 m, bottom-water temperatures between −0.06 and 0.21 ∘C, and salinities between 34.8 and 34.89 (Jennings et al., 2004; Rytter et al. (2002). Rytter et al. (2002) suggest that I. norcrossi may be influenced by the high surface productivity at oceanographic fronts. In conclusion, I. norcrossi is a shelf to upper slope species that seems to prefer relatively stable salinities of 34.8–34.9, in regions where winter cooling of Atlantic Water and mixing with fresher and colder Polar Water result in formation of Arctic Water masses, particularly to the north of Iceland, where Arctic Intermediate Water is formed by ocean convection.
Long-standing confusion and inconsistencies in the literature regarding the identification and grouping of species (e.g. Hunt and Corliss, 1993; Scott et al., 2008), sometimes even within the same study (e.g. Korsun and Hald, 1998), obscure ecological information, profoundly hindering the use of foraminifera as dependable palaeoenvironmental proxies. The development of online databases such as the World Register of Marine Species (WORMS, http://www.marinespecies.org, last access: 19 October 2020), the World Foraminifera Database (http://www.marinespecies.org/foraminifera/, last access: 19 October 2020), and the Global Biodiversity Information Facility (https://www.gbif.org/species, last access: 19 October 2020) has opened up access to species images and accepted taxonomy; however, original species descriptions (accessible by subscription to the Ellis and Messina catalogue; http://www.micropress.org/em/about.php, last access: 27 April 2020) are rarely included in the database resources and can be difficult to source. This often leaves researchers to identify specimens through figures alone, and this can propagate taxonomic errors as some online databases (as with literature) are anecdotally known to have incorrect denominations, and included images should be approached with caution.
By providing clear guidance on the correct species identifications for C. laevigata, C. neoteretis, C. teretis, P. neocarinata, I. helenae, and I. norcrossi, we aim to curtail the misidentification of these morphologically similar species, elucidate ecological affinities, and thus improve accurate palaeoenvironmental reconstructions based on the differing environmental preferences of examined taxa. For example, misidentifying I. helenae as C. neoteretis in a palaeo-context would result in reconstructing a past environment reflecting chilled warmer Atlantic water rather than a cold, lower-salinity water mass or sea ice marginal settings (Table 2). Similarly, misidentifying a fossil C. neoteretis for a C. laevigata would suggest a boreal environment with warm Atlantic waters (e.g. Mackensen and Hald, 1988) rather than a polar to cold boreal environment, with modified and cooled Atlantic Water (e.g. Knudsen et al., 2012). Additionally, due to differences in vital effects of different genera and species (Kristjánsdóttir et al., 2007), it would be detrimental to mix different genera and species when analysing their geochemical signal. Therefore, it is of utmost importance that care and diligence be employed when identifying morphologically similar taxa.
Table 2Ecological preferences for Cassidulina laevigata, Cassidulina neoteretis, Paracassidulina neocarinata, Islandiella helenae, and Islandiella norcrossi. Since Cassidulina teretis is extinct, we cannot provide modern environmental preferences for this species, but rely on findings from palaeo studies.
Here, we discuss the origin of some of the persistent taxonomic issues affecting these species and provide guidance on how researchers can identify these key Arctic–North Atlantic foraminiferal taxa. This approach will clarify ecological affinities and produce more accurate palaeoenvironmental reconstructions.
3.1 Separation of genera: Cassidulina, Paracassidulina, and Islandiella
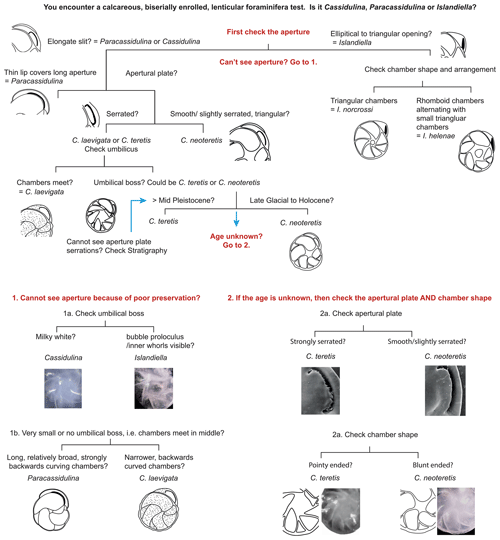
The easiest way to separate the three genera is through observation of their apertures, which are always placed on the side of the test. For Cassidulina, the aperture is a narrow slit with an apertural plate, whereas for Islandiella, the aperture is a broader triangular to elliptical opening on the margin of the final chamber (Table 1) with a free apertural tongue protruding from the interior of the chamber. The apertural slit of Paracassidulina is much longer than that for Cassidulina, and instead of a regular apertural plate it has a narrow edge. These features are even more easily recognized if the last chamber is broken off.
Another, secondary line of taxonomic distinction is the colour difference between Cassidulina and Islandiella. Islandiella normally exhibits a “cold” white colour, and well-preserved tests are transparent to translucent due to the radial wall type, whereas the granular wall type of Cassidulina most commonly results in a “warmer” white colour and translucent to opaque tests in well-preserved specimens. However, we recommend caution in using colour and transparency as diagnostic tools, as poor preservation and subjectivity may lead to misidentification, though they may be used as secondary characteristics. The difference in wall type also causes the umbilical area of Cassidulina to be non-translucent and milky white, while in Islandiella the umbilical area is translucent, allowing a view to earlier whorls and the proloculus in well-preserved specimens.
Additionally, testing whether the wall structure is granular (as with Cassidulina and Paracassidulina) or radial (as with Islandiella) can be achieved by breaking the specimens and investigating pieces of them in polarized light. This may not be a practical approach when investigating many specimens but is a good option when testing selected individuals that are representative for a group of specimens. It is noteworthy that C. neoteretis, due to its granular wall structure, is much more prone to CaCO3 dissolution than are I. helenae and I. norcrossi (Anne Jennings, personal observation, 2020).
3.2 Guide to distinguishing among species of Cassidulina
Parker and Jones (1865) and Mackensen and Hald (1988) exemplify the potential taxonomic confusion surrounding C. laevigata and other species from morphologically comparable taxa of Cassidulina or Islandiella. Plate XV (figs. 1–4) in Parker and Jones (1865) shows illustrations of “C. laevigata” from Baffin Bay, which appear to have clear umbilical boss areas; however, C. laevigata is characterized by long and curved chambers meeting in the centre, resulting in the absence of an umbilical boss (Fig. 1). We therefore suggest that Parker and Jones (1865) misidentified the specimen, which is more likely C. neoteretis or I. helenae (the quality of drawing of the aperture makes it difficult to distinguish). The C. laevigata illustrated on plate XVII by Parker and Jones (1865) from the Atlantic realm also appears morphologically different to the arctic C. laevigata on their plate XV, and the absence of an umbilical boss region in the sample suggests that two different designations are represented within the same study. The apertures portrayed in the Parker and Jones (1865) C. laevigata illustrations (plate XV, figs. 1–4; plate XVII, fig. 64a–c) lack sufficient detail to confidently assign specific species. This misidentification permeated through the literature (e.g. Eade, 1967; Belanger and Streeter, 1980; Jansen et al., 1983; Mackensen et al., 1985) until Mackensen and Hald (1988) provided criteria for separating C. laevigata and C. teretis (later re-allocated to C. neoteretis by Seidenkrantz (1995) based on the umbilical area, pores, and apertures).
Cassidulina teretis and Cassidulina neoteretis
We provide guidance on how to separate the species C. neoteretis and C. teretis in Table 1, Sect. 3.5, and Fig. 6. Seidenkrantz's (1995) erection of C. neoteretis as a new species distinct from C. teretis emphasized the importance of the extinction of C. teretis as a biostratigraphic marker for the Neogene and early to mid-Pleistocene of the North Atlantic. However, as pointed out by Seidenkrantz (1995), the disappearance of C. teretis seems to be time transgressive with a later disappearance of C. teretis in the Arctic Ocean than in the North Atlantic. Lazar et al. (2016) showed that C. teretis first disappeared in Marine Isotope Stage 3 in part of the Arctic Ocean, indicating that further work is needed to establish this species as a regional stratigraphical marker. Lazar et al. (2016) suggested that C. neoteretis could be an ecophenotype of C. teretis, which has potentially evolved its apertural plate in response to changing diet; however, as C. teretis has not yet been found in any modern sediments and appears to be extinct, this issue cannot currently be resolved through genetic analyses. Furthermore, as the extinct C. teretis may have slightly different ecologies (C. teretis is found in more shallow-water shelf palaeoenvironments than C. neoteretis; see Table 2 and above), the two species should still be separated whenever possible. We agree with the conclusion of Lazar et al. (2016) that separating C. teretis and C. neoteretis on morphological evidence alone can at times be difficult. However, although Lazar et al. (2016) showed that some temporal overlap may occur, in most studies (temporal intervals) all specimens will belong to either C. teretis or C. neoteretis. Consequently, it normally will not be necessary to investigate all specimens in detail to separate them. Instead, it is sufficient to investigate the best-preserved specimens to identify which of the two species is present. Currently, it seems unproblematic to assign the name C. teretis to all Pliocene and likely also older Pleistocene specimens (e.g. Saher et al., 2012) and C. neoteretis to late Quaternary and certainly to last glacial and Holocene specimens. However, a more precise evaluation of the temporal shift from C. teretis to C. neoteretis at different locations would be very valuable.
3.3 Islandiella helenae and Islandiella norcrossi: dangers of grouping
Though easily differentiated despite their similarities (Table 1, Figs. 5 and 6), I. helenae and I. norcrossi have frequently been grouped into one category (e.g. Hunt and Corliss, 1993; Korsun and Hald, 1998; Polyak et al. 2002; Ovsepyan and Taldenkova, 2019). At times, intermediate forms can be seen (e.g. Fig. 4g and Ovsepyan and Taldenkova, 2019 pl. 2, figs. 4–6), yet most commonly their chamber arrangements are remarkably different and the rare studies that distinguish between these two taxa demonstrate distinct environmental preferences (Vilks et al., 1982; Korsun and Hald, 1998; Jennings and Helgadóttir, 1994). For example, Hunt and Corliss (1993), working on surface sediments in the Canadian Arctic Archipelago, grouped I. norcrossi within I. helenae due to stated difficulties in distinguishing large specimens between either taxon. Hald and Korsun (1997) and Korsun and Hald (1998) report occurrences of I. helenae but group it into an I. norcrossi s.l. (sensu lato) category. Korsun and Hald (2000) discussed environmental preferences of an I. norcrossi group that included “mostly the helenae form” despite the majority of the Islandiella specimens being identified and reported as I. helenae in their results. The consistent grouping in the literature of I. helenae and I. norcrossi in particular has obscured their ecological preferences and environmental distributions (e.g. Hunt and Corliss, 1993). In turn, this limited understanding of environmental preferences has promoted continued grouping, leading to a somewhat circular argument for the collective treatment of these taxa (e.g. Hanslik and Hermelin, 2011).
3.4 Propagation of taxonomic confusion in recent literature
Taxa from the genera Cassidulina, Paracassidulina, and Islandiella continue to be either misidentified or grouped together in the literature due to perceived difficulties in taxonomic separation based on test morphology or a failure to recognize their distinct environmental preferences. For example, Scott et al. (2008, 2009) and Schell et al. (2008) consistently used the term Islandiella teretis (Tappan) for the combined group of I. helenae, I. norcrossi, C. neoteretis, and C. laevigata, considering I. teretis to be a species characterized by “apertural variability”. The I. teretis illustrated by Vilks (1969) is likely a specimen of C. neoteretis. However, Vilks later considered I. teretis (cf. Vilks and Rashid, 1976; Vilks et al., 1979) to encompass I. helenae, and subsequently grouped C. laevigata, I. teretis and I. helenae within I. helenae Feyling-Hanssen and Buzas (Vilks, 1989). Belanger and Streeter (1980) regarded I. helenae to be a shallower water homeomorph of C. teretis (i.e. C. neoteretis) and suggested that most specimens in the literature were I. helenae. Indeed, misunderstandings concerning the distinction between I. helenae, I. norcrossi, and C. neoteretis have confused their proper identifications, especially in the Canadian Arctic (e.g. Scott et al., 2008). In addition, Hanslik and Hermelin (2011) did not differentiate between specimens of Cassidulina and Islandiella, grouping these two taxa as C. neoteretis (presumably due to the morphological similarities between these species and the more regular occurrence of C. neoteretis with respect to I. helenae and I. norcrossi). Such a grouping has serious implications for accurate environmental reconstructions, since C. neoteretis and the two Islandiella species have very different environmental affinities (Table 2). Cronin et al. (2019) further complicated the taxonomic issue regarding arctic Cassidulina by reassigning species already identified by authors as either Islandiella (e.g. Farmer et al., 2011) or C. neoteretis (cf. Keigwin et al., 2018) as C. teretis without clear criteria as to how this reassignment was justified. This is particularly problematic as some specimens in Cronin et al.'s (2019) plate 2 have been identified as C. teretis, despite showing apertures and chamber arrangements indicative of I. helenae (plate 2, fig. 4) and I. norcrossi (plate 2, fig. 3). These taxonomic errors, complete with images of the foraminifers, become embedded in the literature and thus continue to confuse later workers reliant on previous work, resulting in the propagation of taxonomic uncertainties and flawed understanding of the environmental preferences of individual species. In order to inhibit error propagation, we recommend that authors clarify their own discernment of species criteria in relevant material and methods (including images) or taxonomic notes.
3.5 Guidance for identifying similar morphotypes of Cassidulina, Paracassidulina, and Islandiella
We suggest that with careful examination of key diagnostic features such as chamber arrangements and apertures, supplemented by determination of test microstructure, species of the genera Cassidulina, Paracassidulina, and Islandiella can be separated with confidence, as they are separate species with different environmental preferences (Table 2). Table 1 and Fig. 5 illustrate key taxonomic differences between the studied Cassidulina, Paracassidulina, and Islandiella species, and we also provide a decision tree (Fig. 6) to aid species identification of taxa from these three groups.
Nevertheless, we recognize that there are instances where the correct assignment of species identification can be problematic: for example, when there appears to exist gradation between species (e.g. Rytter et al., 2002), where specimens have undergone taphonomic damage, or where assemblages are typically composed of smaller test sizes (<125 µm), in arctic shelf environments for example (Pieńkowski et al., 2014; Husum et al., 2015). In these cases, we suggest that species are assigned the group name of the dominant taxon, thereby acknowledging the ambiguity of the identification; for example, in a sample where Islandiella is dominated by I. norcrossi, specimens which cannot be confidently assigned to this taxon should be grouped as Islandiella spp. followed by naming the most dominant species in brackets. Another problematic taxonomic issue is the separation of C. teretis and C. neoteretis, as discussed earlier. Lazar et al. (2016) point out that the use of the apertural plate serration to distinguish the two species (as illustrated by Seidenkrantz, 1995) hinges on access to high-resolution microscopy, which is not always available or practical. However, the serration is visible in regular light microscopy in well-preserved specimens, and we suggest that, whenever possible, the serration of the apertural plate and the sharpness of the margin (with C. neoteretis displaying a sharper margin than C. teretis) should be used to separate these two taxa. In cases where such an approach is impractical or impossible, and if the actual stratigraphical level is in the transitional time window for the two species (i.e. around the mid-Pleistocene), specimens should be reported as C. teretis/C. neoteretis.
The historical and persistent grouping of morphologically similar benthic foraminiferal taxa belonging to Cassidulina, Paracassidulina, or Islandiella, which are important environmental indicator species in Atlantic and arctic environments, has resulted in uncertainties both in their taxonomic separation and ecological preferences. It is vital that care is taken to separate these taxa to ensure well-founded environmental reconstructions. Our paper brings together, for the first time, the original descriptions of these taxa supplemented by taxonomical notes and provides illustrations and clear guidelines necessary for confident species identification and taxa separation. It is our intention that this resource will benefit the micropalaeontological community and serve to develop a better understanding of the taxonomy and ecological affinities of these important North Atlantic and arctic benthic foraminiferal species.
We suggest that in the first instance, the aperture should be used to separate specimens of Cassidulina, Paracassidulina, and Islandiella, and chamber arrangement should be used to identify to species level within each of the three genera, although the aperture may also be used to distinguish between various species of Cassidulina. In addition, the microstructure is an important character for the separation of these genera, Islandiella being radial and Paracassidulina and Cassidulina being granular. As the six species discussed here have significant differences in their ecological preferences, a correct determination of the different species within these three genera will result in improved palaeoenvironmental reconstructions in the Arctic and North Atlantic realm.
No data sets were used in this article.
Table S1 provides sample details for specimens illustrated in Figs 1–5. Table S2 provides sample details and information on holotypes, paratypes and syntypes shown in Fig. 5. The supplement related to this article is available online at: https://doi.org/10.5194/jm-40-37-2021-supplement.
AGC and AJP conceptualized the paper and prepared the manuscript with contributions from all the authors. AJP created the figures and Table S1. AGC and AJ created the decision tree. All authors contributed to all aspects of the manuscript (text, photographs, and tables).
The authors declare that they have no conflict of interest.
We thank Mark Furze for help with figures, while some photographs were taken with the aid of Svend Meldgaard Christiansen†. We also thank Jeremy Lloyd for providing the photograph of I. norcrossi for Fig. 4g. We are grateful to the Smithsonian Libraries and the Cushman Foundation for Foraminiferal Research for allowing the use of original illustrations and re-images of the taxa of interest. We also thank Naima El Bani Altuna for help with translations. We thank Joachim Schönfeld, Hugh Grenfell, and Kirsty Edgar, whose comments improved the manuscript. Kirsty Edgar is also thanked for her editorial handling of our manuscript.
This research was funded by a Systematics Research Fund (Linnean Society of London and Systematics Association) awarded to Anna J. Pieńkowski and Alix G. Cage, along with a Marie Curie Career Integration Grant (FP7-PEOPLE-2011-CIG304178) and an NSERC Discovery Grant (RGPIN-2016-05457) awarded to Anna J. Pieńkowski. Marit-Solveig Seidenkrantz was funded through the Independent Research Fund Denmark/Natural Science project G-Ice (project no 7014–00113B) with additional funding from the European Union's Horizon 2020 research and innovation program under Grant Agreement No. 869383 (ECOTIP). Funding for Anne Jenning's participation comes from National Science Foundation OPP 1804504 “Timing and Paleoceanographic Impacts of the Onset of Arctic-Baffin Bay Throughflow”.
This paper was edited by Kirsty Edgar and reviewed by Joachim Schönfeld and Hugh Grenfell.
Alve, E.: Benthic foraminiferal responses to absence of fresh phytodetritus: a two-year experiment, Mar. Micropaleontol., 76, 67–75, 2010.
Belanger, P. E. and Streeter, S. S.: Distribution and ecology of benthic foraminifera in the Norwegian-Greenland Sea, Mar. Micropaleontol., 5, 401–428, 1980.
Brady, H. B.: XL. – On some Arctic Foraminifera from soundings obtained on the Austro-Hungarian North-Polar Expedition of 1872–1874, Ann. Mag. Nat. Hist., 5, 393–418, 1881.
Brady, H. B.: Report on the foraminifera dredged by H.M.S. Challenger, during the years 1873-1876, in: Report on the scientific results of the voyage of H.M.S. Challenger, during the years 1873-1876, Zoology, edited by: Murray, J., Edinburgh, Neill and Company, 1–814, 1884.
Cage, A. G.: The Modern and Late Holocene Marine Environments of Loch Sunart, N.W. Scotland, PhD thesis, University of St Andrews, Scotland, 399 pp., 2005.
Christiansen, C. F.: Changes in ocean currents and climate during the Holocene, based on benthic foraminifera, MSc thesis, Department of Biology, University of Southern Denmark & Department of Geoscience, Aarhus University, 61 pp, 2018.
Conradsen, K., Bergsten, H., Knudsen, K. L., Nordberg, K., and Seidenkrantz, M.-S.: Recent benthic foraminiferal distribution in the Kattegat and the Skagerrak, Scandinavia, Cushman Foundation Special Publication, 32, 53–68, 1994.
Cronin, T. M., Seidenstein, J., Keller, K., McDougall, K., Ruefer, A., and Gemery, L.: The benthic foraminifera Cassidulina from the Arctic Ocean: application to paleoceanography and biostratigraphy, Micropaleontology, 65, 105–125, 2019.
Cushman, J. A.: The Foraminifera of the Atlantic Ocean: United States National Museum, Bulletin no. 104, pt. 3, 1–149, 1922.
Cushman, J. A.: The Foraminifera of the Atlantic Ocean, Part 7, Nonionidae, Camerinidae, Peneroplidae and Alveolinellidae, Bulletin of the United States National Museum, 104, 1–79, 1930.
Cushman, J. A.: New arctic foraminifera collected by Capt. R. A. Bartlett from Fox Basin and off the northeast coast of Greenland, Smithsonian Miscellaneous Collections, 89, 1–8, 1933.
Delage, Y. and Herouard, E.: Traité de Zoologie Concrète, Tome I, La Cellule et les Protozoaires. Schleicher Frères, Paris, 1–584, 1896 (in French).
d'Orbigny, A. D.: Tableau méthodique de la classe des Céphalopodes, Ann. Sci. Nat., 7, 245–314, 1826 (in French).
d'Orbigny, A. D.: Foraminifères, in: de la Sagra R., Histoire physique, politique et naturelle de l'ile de Cuba, edited by: Bertrand, A., 1–224, 1839 (in French).
Eade, J. V.: New Zealand recent Foraminifera of the families Islandiellidae and Cassidulinidae, New Zeal. J. Mar. Fresh., 1, 421–454, 1967.
Eade, J. V.: Globocassidulina nipponensis, new name for Cassidulina orientale Cushman, 1925 preoccupied, Contributions from the Cushman Foundation for Foraminiferal Research, 20, 65–66, 1969.
Ellis, B. F. and Messina, A.: Catalogue of Foraminifera, American Museum of Natural History and Micropaleontology Press, New York, 1949 and update supplements up to and including 2019, available at: http://www.micropress.org/em/about.php (last access: 27 April 2020), 1949.
Farmer, J. R., Cronin, T. M., de Vernal, A., Dwyer, G. S., Keigwin, L. D., and Thunell, R. C.: Ocean temperature variability in the western Arctic Ocean during the last 8000 years, Geophys. Res. Lett., 38, l24602, https://doi.org/10.1029/2011gl049714, 2011.
Feyling-Hanssen, R. W.: The stratigraphy of the Quaternary Clyde Foreland Formation, Baffin Island, illustrated by the distribution of benthic foraminifera, Boreas, 5, 77–94, 1976a.
Feyling-Hanssen, R. W.: The Clyde Foreland Formation: A micropaleontological study of Quaternary stratigraphy, 1st International Symposium on Benthonic Foraminifera of Continental Margins, Part B: Paleoecology and Biostratigraphy, Maritime Sediments, Special Publication, 1, 315–377, 1976b.
Feyling-Hanssen, R. W.: Microbiostratigraphy of young Cenozoic marine deposits of the Quivituq Peninsula, Baffin Island, Mar. Micropaleontol., 5, 153–184, 1980a.
Feyling-Hanssen, R. W.: An assemblage of Pleistocene foraminifera from Pigojoat, Baffin Island, J. Foramin. Res., 10, 266–285, 1980b.
Feyling-Hanssen, R. W.: Foraminiferal stratigraphy in the Plio-Pleistocene Kap København Formation, North Greenland, Meddelelser om Grønland, Geoscience 24, 1–32, 1990.
Feyling-Hansen, R. W. and Buzas, M. A.: Emendation of Cassidulina and Islandiella helenae new species, J. Foramin. Res., 6, 154–158, 1976.
Feyling-Hanssen, R. W., Jórgensen, J. A, Knudsen, K. L., and Andersen, A. L.: Late Quaternary foraminifera from Vendsyssel, Denmark and Sandes, Norway, Geol. Soc. Denmark Bull., 21, 67–71, 1971.
Feyling-Hanssen, R. W., Funder, S., and Petersen, K. S.: The Lodin Elv Formation; a Plio-Pleistocene occurrence in Greenland, B. Geol. Soc. Denmark, 31, 81–106, 1983.
Gooday, A. J. and Lambshead, P. J. D.: Influence of seasonally deposited phytodetritus on benthic foraminiferal populations in the bathyal northeast Atlantic: the species response, Mar. Ecol. Prog. Ser., 58, 53–67, 1989.
Green, K. E.: Ecology of some Arctic foraminifera, Micropaleontology, 6, 57–78, 1960.
Gudina, V. I.: Foraminifera and stratigraphy of the Northwest Siberian Quaternary, Akademia Nauk SSSR, Siberian Department, Institute of Geology and Geophysics U.D.K. 563.12 (119) (571.1), 1–132, 1966 (in Russian).
Gudina, V. I.: The marine Pleistocene of Siberian lowlands, Foraminifera of the north part of Yenisei lowlands, Akademia Nauk SSSR, Siberian Department, Institute of Geology and Geophysics, 63, 1–80, 1969.
Hald, M. and Korsun, S.: Distribution of modern benthic foraminifera from fjords of Svalbard, Eur. Arctic, J. Foram. Res., 27, 101–122, 1997.
Hald, M. and Steinsund, P. I.: Benthic foraminifera and carbonate dissolution in the surface sediments of the Barents and Kara Sea, in: Surface-sediment composition and sedimentary processes in central Arctic Ocean and along the Eurasian Continental Margin, edited by: Stein, R., Ivanov, G. I., Levitan, M. A., and Fahl, K., Berichte zur Polarforschung, 212, 285–307, 1996.
Hansen, K. E., Giraudeau, J., Wacker, L., Pearce, C., and Seidenkrantz, M.-S.: Reconstruction of Holocene oceanographic conditions in the Eastern Baffin Bay, Clim. Past, 16, 1075–1095, 2020.
Hanslik, D. and Hermelin, O.: Late Quaternary benthic foraminiferal assemblages from the central Arctic Ocean, in: Late Quaternary Biostratigraphy and Paleoceanography of the Central Arctic Ocean, PhD thesis, Stockholm University, Sweden, 32 pp., 2011.
Hunt, A. S. and Corliss, B. H.: Distribution and microhabitats of living (stained) benthic foraminifera from the Canadian Arctic Archipelago, Mar. Micropaleontol., 20, 321–345, 1993.
Husum, K., Hald, M., Stein, R., and Weißschnur, M: Recent benthic foraminifera in the Arctic Ocean and Kara Sea continental margin, Arktos, 1, 5, https://doi.org/10.1007/s41063-015-0005-9, 2015.
Jansen, E., Sejrup, H. P, Fjæran, T., Hald, M. Holtedahi, H., and Skarbø, O.: Late Weichselian paleoceanography of the southeastern Norwegian Sea, Norsk Geol. Tidsskr., 63, 117–146, 1983.
Jansen, E., Sjøholm, J., Bleil, U., and Erichsen, J. A.: Neogene and Pleistocene glaciations in the northern hemisphere and Late Miocene-Pliocene global ice volume fluctuations: Evidence from the Norwegian Sea, in: Geological History of the Polar Oceans: Arctic versus Antarctic, edited by: Bleil, U. and Thiede, J., Kluwer Academic Publishers, the Netherlands, 677–705, 1990.
Jennings, A. E. and Helgadóttir, G.: Foraminiferal assemblages from the fjords and shelf of Eastern Greenland, J. Foramin. Res., 24, 123–144, 1994.
Jennings, A., Weiner, N. J., Helgadottir, G., and Andrews, J. T.: Modern foraminiferal faunas of the SW to N Iceland Shelf: Oceanographic and environmental controls, J. Foramin. Res., 34, 180–207, 2004.
Jennings, A., Andrews, J. T., Reilly, B., Walczak, M., Jakobsson, M., Mix, A., Stoner, J., Nicholls, K. W., and Cheseby, M. Modern foraminiferal assemblages in northern Nares Strait, Petermann Fjord, and beneath Petermann Ice Tongue, NW Greenland, Arct. Antarct. Alp. Res., 52, 491–511, 2020.
Jennings, A. E., Andrews, J. T., and Wilson, L.: Holocene environmental evolution of the SE Greenland shelf North and South of the Denmark Strait: Irminger and East Greenland Current interactions, Quaternary Sci. Rev., 30, 980–998, 2011.
Jones, R. W.: The Challenger Foraminifer, Oxford University Press, Oxford, 151 pp., 1994.
Keigwin, L., Klotsko, S., Zhao, N., Reilly, B. Giosan, L., and Driscoll, N. W.: Deglacial floods in the Beaufort Sea preceded Younger Dryas cooling, Nat. Geosci., 11, 599–604, 2018.
Klitgaard-Kristensen, D., Sejrup, H. P., and Halflidason, H.: Distribution of recent calcareous benthic foraminifera in the northern North Sea and relation to the environment, Polar Res., 21, 275–282, 2002.
Knudsen, K. L. and Ásbjörnsdóttir, L.: Plio-Pleistocene foraminiferal stratigraphy and correlation in the Central North Sea, Mar. Geol., 101, 113–124, 1991.
Knudsen, K. L., Eiríksson, J., and Bartels-Jónsdóttir, H. B.: Oceanographic changes through the last millennium off North Iceland: temperature and salinity reconstructions based on foraminifera and stable isotopes, Mar. Micropaleontol., 84–85, 54–73, 2012.
Korsun, S. and Hald, M.: Modern Benthic Foraminifera off Novaya Zemlya tidewater glaciers, Russian Arctic, Arct. Antarct. Alp. Res., 30, 61–77, 1998.
Korsun, S. and Hald, M.: Seasonal dynamics of benthic foraminifera in a glacially fed fjord of Svalbard, Eur. Arctic, J. Foram. Res., 30, 251–271, 2000.
Korsun, S. A. and Polyak, L. V.: Distribution of benthic foraminiferal morphogroups in the Barents Sea, Oceanology, 29, 838–844, 1989.
Korsun, S. A., Pogodina, I. A., Forman, S. L., and Lubinski, D. J.: Recent foraminifera in glaciomarine sediments from three arctic fjords of Novaja Zemlja and Svalbard, Polar Res., 14, 15–32, 1995.
Kristjánsdóttir, G. B., Lea, D. W., Jennings, A. E., Pak, D. K., and Belanger, C.: New spatial Mg/Ca-temperature calibrations for three Arctic, benthic foraminifera and reconstruction of north Iceland shelf temperature for the past 4000 years, Geochem. Geophys. Geosys., 8, Q03P21, https://doi.org/10.1029/2006GC001425, 2007.
Lagoe, M. B.: Recent benthic foraminifera from the central Arctic Ocean, J. Foram. Res., 7, 106–130, 1977.
Lagoe, M. B.: Recent Arctic foraminifera: an overview, paper presented at the Quaternary Depositional Environments of the Pacific Coast, Pacific Coast Paleoceanography Symposium 4, Pacific Section, Soc. Econ. Pa., Los Angeles, California, 9 April 1980, 33–42, 1980.
Laursen, G. V., Heilmann-Clausen, C., and Thomsen, E.: Cenozoic stratigraphy of the eastern North Sea based on foraminifera, dinoflagellates, and calcareous nannofossils, Final biostratigraphical report on the CENOS-project, Unpublished report, Department of Earth Sciences, Aarhus University, Aarhus, 1992.
Lazar, K. B., Polynak, L., and Dipre, G.: Re-examination of the use of Cassidulina neoteretis as a Pleistocene biostratigraphic marker in the Arctic Ocean, J. Foramin. Res., 46, 115–123, 2016.
Lloyd, J. M.: Modern distribution of benthic foraminifera from Disko Bugt, West Greenland, J. Foramin. Res., 36, 315–331, 2006.
Loeblich, A. R. and Tappan, H.: Studies of Arctic Foraminifera, Smithsonian Miscellaneous Collections, 121, 1–150, 1953.
Loeblich, A. R. and Tappan, H.: Foraminiferal Genera and their Classification, Van Nostrand Reinhold Company, New York, 970 pp., 1987.
Mackensen, A.: Bentische Foraminiferen aus dem Island-Schottland Riicken: Umwelt-Anzeiger an der Grenze zweier ozeanischer Raume, Paliiontologische Zeitschrift, Stuttgart, 61, 149–119, 1987 (in German).
Mackensen, A. and Hald, M.: Cassidulina teretis Tappan and C. laevigata d'Orbigny. Their Modern and Late Quaternary distribution in northern seas, J Foramin. Res., 18, 16–24, 1988.
Mackensen, A., Sejrup, H. P., and Jansen, E.: The distribution of living benthic foraminifera on the continental slope and rise off southwest Norway, Mar. Micropaleontol., 9, 275–306, 1985.
Milker, Y. and Schmiedl, G.: A taxonomic guide to modern benthic shelf foraminifera of the western Mediterranean Sea, Palaeontol. Electron., 15 15.2.16A, https://doi.org/10.26879/271, 2012.
Möller, P., Fedorov, G., Pavlov, M., Seidenkrantz, M.-S., and Sparrenbom, C.: Glacial and palaeoenvironmental history of the Cape Chelyuskin area, Arctic Russia., Polar Res., 27, 222–248, 2008.
Murray, J. W.: An atlas of British Recent foraminiferids, Elsevier Publishing Company Incorporated, New York, USA, 244 pp., 1971.
Murray, J. W.: An Illustrated Guide to the Benthic Foraminifera of the Hebridean Shelf, West of Scotland, with notes on their mode of life. Palaeontol. Electron., 5, 1–31, 2003.
Mudie, P. J., Keen, C. E., Hardy, I. A., and Vilks, G.: Multivariate analysis and quantitative paleoecology of benthic foraminifera in recent and Late Quaternary sediments, northern Canada, Mar. Micropaleontol., 8, 283–313, 1984.
Nomura, R.: Cassidulinidae (foraminiferida) from the uppermost Cenozoic of Japan (part 1), Tohoku University Scientific Reports, 2nd Series (Geology), 53, 1–101, 1983.
Nørvang, A: The Zoology of Iceland, Foraminifera. Copenhagen & Reykjavik: Ejnar Munksgaard, v. 2, Part 2: 1–79, 1945.
Nørvang, A.: Islandiella n.g. and Cassidulina d'Orbigny, Vidensk. Medd. Dansk Naturhist. Foren. 120, 25–41, 1958.
Osterman, L. E., Poore, R. Z., and Foley, K. M.: Distribution of benthic foraminifers (>125 µm) in the surface sediments of the Arctic Ocean, U.S. Geol. Surv. Bull., 2164, 1–28, 1999.
Ovsepyanm Y. S, and Taldenkova, E. E.: Distribution Patterns and Morphology of Islandiella norcrossi (Cushman) in the Upper Quaternary Deposits of the Laptev Sea, Paleontol. J., 53, 10–19, 2019.
Parker, F. L.: Distribution of the foraminifera in the northwest Gulf of Mexico, Bulletin of the Museum of Comparative Zoology, 111, 453–588, 1954.
Parker, W. K. and Jones, T. R.: On some foraminifera from the North Atlantic and Arctic Oceans, including Davis Straits and Baffin Bay, Philos. T. R. Soc. Lond., 155, 325–441, 1865.
Perner, K., Moros, M., Jennings, A., Lloyd, J. M., and Knudsen, K. L.: Holocene palaeoceanographic evolution of West Greenland, Holocene 23, 374–387, 2013.
Perner, K. Moros, M., Lloyd, J. M., Jansen, E., and Stein, R.: Mid to late Holocene strengthening of the East Greenland Current linked to warm subsurface Atlantic water, Quaternary Sci. Rev., 129, 296–307, 2015.
Phleger, F. B: Ecology of Foraminifera, Northwest Gulf of Mexico, Part I. Foraminifera Distribution, The Geological Society of America, Memoir, 46, 1–88, 1951.
Phleger, F. B. and Parker, F. L.: Ecology of Foraminifera, Northwest Gulf of Mexico, Part II. Foraminifera Species, The Geological Society of America, Memoir, 46, 1–64, 1951.
Phleger, F. B., Parker, F. L., and Pierson, J.: North Atlantic foraminifera, Reports of the Swedish Deep Sea Expedition 1947–1948, 7, 3–122, 1953.
Piénkowski, A. J., England, J. H., Furze, M. F. A., MacLean, B., and Blasco, S.: The late Quaternary environmental evolution of marine Arctic Canada: Barrow Strait to Lancaster Sound, Quaternary Sci. Rev., 91, 184–203, 2014.
Platon, E.: Benthic Foraminifera in Two Stressed Environments of the Northern Gulf of Mexico, LSU Historical Dissertations and Theses, 307, Louisiana State University and Agricultural and Mechanical College, available at: https://digitalcommons.lsu.edu/gradschool_disstheses/307, 291 pp, 2001.
Poag, C. W.: Benthic Foraminifera of the Gulf of Mexico: distribution, ecology, paleoecology, Texas A&M University Press, College Station, 240 pp., 2015.
Polovodova Asteman, I. and Nordberg, K.: Foraminiferal fauna from a deep basin in Gullmar Fjord: The influence of seasonal hypoxia and North Atlantic Oscillation, J. Sea Res., 79, 40–49, 2013.
Polyak, L., Korsun, S., Febo, L. A., Stanovoy, V., Khusid, T., Hald, M., Paulsen, B. E., and Lubinski, D. J.: Benthic foraminiferal assemblages from the southern Kara Sea, a river influenced arctic marine environment, J. Foramin. Res., 32, 252–273, 2002.
Polyak, L., Best, K. M., Crawford, K. A., Council, E. A., and St-Onge, G.: Quaternary history of sea ice in the western Arctic Ocean based on foraminifera, Quaternary Sci. Rev., 79, 145–156, 2013.
Rodrigues, C. G., Hooper, K., and Jones, P.C.: The apertural structures of Islandiella and Cassidulina, J. Foramin. Res., 10, 48–60, 1980.
Rytter, F., Knudsen, K. L., Seidenkrantz, M.-S., and Eiríksson, J.: Modern distribution of benthic foraminifera on the North Icelandic shelf and slope, J. Foramin. Res., 32, 217–244, 2002.
Saher, M., Klitgaard Kristensen, D., Hald, M., Pavlova, O., and Jørgensen, L. L.: Changes in distribution of calcareous benthic foraminifera in the central Barents Sea between the periods 1965–1992 and 2005–2006, Global Planet. Change, 98–99, 81–96, 2012.
Sejrup, H. P. and Guilbault, J. P.: Cassidulina reniforme and C. obtusa (foraminifera), taxonomy, distribution and ecology, Sarsia, 65, 79–85, 1980.
Sejrup, H. P., Fjaeran, T., Hald, M., Beck, L., Hagen, J., Miljeteig, I., Morvik, I., and Norvik. O.: Benthonic foraminifera in surface samples from the Norwegian continental margin between 62∘ and 65∘ N, J. Foramin. Res., 11, 277–295, 1981.
Schell, T. M., Moss, T. J., Scott, D. B., and Rochon, A.: Paleo-sea ice conditions of the Amundsen Gulf, Canadian Arctic Archipelago: Implications from the foraminiferal record of the last 200 years, J. Geophys. Res., 113, C03S02, https://doi.org/10.1029/2007JC004202, 2008.
Schmiedl, G., Mackensen, A., and Müller, P. J.: Recent benthic foraminifera from the eastern South Atlantic Ocean: Dependence on food supply and water masses, Mar. Micropaleontol., 32, 249–287, 1997.
Schröder-Adams, C. J. and van Rooyen, D.: Response of Recent Benthic Foraminiferal Assemblages to Contrasting Environments in Baffin Bay and the Northern Labrador Sea, Northwest Atlantic, Arctic, 64, 317–341, 2011.
Scott, D. B., Takayanagi, Y., Hasegawa, S., and Saito, T.: Illustration and re-evaluation of affinities of Neogene foraminifera described From Japan, Palaeontol. Electron., 3, 1–41 2000.
Scott, D. B., Schell, T., Rochon, A., and Blasco, S.: Modern benthic foramninifera in the surface sediments of the Beaufort Shelf, Slope and Mackenzie Trough, Beaufort Sea, Canada: taxonomy and summary of surficial distributions, J. Foramin. Res., 38, 228–250, 2008.
Scott, D. B., Schell, T., St-Onge, G., Rochon, A., and Blasco, S.: Foraminiferal assemblage changes over the last 15,000 years on the Mackenzie-Beaufort Sea Slope and Amundsen Gulf, Canada: Implications for past sea ice conditions, Paleoceanography, 24, PA2219, https://doi.org/10.1029/2007PA001575, 2009.
Seidenkrantz, M.-S.: Plio-Pleistocene paleoecology and stratigraphy in the northernmost North Sea, J. Foramin. Res, 22, 363–378, 1992.
Seidenkrantz, M.-S.: Cassidulina teretis Tappan and Cassidulina neoteretis new species (Foraminifera): stratigraphic markers for deep sea and outer shelf areas, J. Micropalaeontol., 14, 145–157, https://doi.org/10.1144/jm.14.2.145, 1995.
Seidenkrantz, M.-S.: Benthic foraminifera as palaeo sea-ice indicators in the subarctic realm: examples from the Labrador Sea – Baffin Bay region, Quaternary Sci. Rev., 79, 135–144, 2013.
Seidenkrantz, M.-S., Ebbesen, H., Aagaard-Sorensen, S., Moros, M., Lloyd, J. M., Olsen, J., Knudsen, M. F., and Kuijpers, A.: Early Holocene large-scale meltwater discharge from Greenland documented by foraminifera and sediment parameters, Palaeogeogr. Palaeocl., 391, 71–81, 2013.
Sen Gupta, B. K. and Aharon, P.: Benthic Foraminifera of bathyal hydrocarbon vents of the Gulf of Mexico. Initial report on communities and stable isotopes, Geo.-Mar. Lett., 14, 88–96, 1994.
Silvestri, A.: Foraminiferi pliocenici della Provincia di Siena; Parte I. Accad. Pont. Nuovi Lincei, Mem., 12, 204 pp., 1896 (in Italian).
Skirbekk, K., Hald, M., Marchitto, T. M., Junttila, J., Klitgaard Kristensen, D., and Aagaard Sørensen, S.: Benthic foraminiferal growth seasons implied from Mg/Ca-temperature correlations for three Arctic species, Geochem. Geophys. Geosy., 17, 4684–4704, 2016.
Steinsund, P. I.: Benthic foraminifera in surface sediments of the Barents and Kara seas: modern and late Quaternary applications, PhD thesis, University of Tromsø, 111 pp, 1994.
Steinsund, P. I., Polyak, L., Hald, M., Mikhailov, V., and Korsun, S.: Distribution of Calcareous Benthic Foraminifera in Recent Sediments of the Barents and Kara Seas, in: Benthic foraminifera in surface sediments of the Barents and Kara seas: modern and late Quaternary applications, edited by: Steinsund, P. I., University of Tromsø, pp. 61–102 (PhD thesis), 1994.
Tappan, H.: Northern Alaska index Foraminifera, Contribution from the Cushman Foundation for Foraminiferal Research, 2, 1–8, 1951.
Thalmann, H. E.: New names and homonyms in Foraminifera. Contribution from the Cushman Foundation for Foraminiferal Research, 1, 41–45, 1950.
Tichenor, H. R.: Foraminifera as indicators of hypoxia off Southwest Pass, Mississippi Delta, Gulf of Mexico?, MSc Thesis, East Carolina University, Greenville, NC, 70 pp., 2013.
Vilks, G.: Recent Foraminifera in the Canadian Arctic, Micropaleontology, 15, 35–60, 1969.
Vilks, G.: Ecology of recent foraminifera on the Canadian continental shelf of the Arctic Ocean, in: The Arctic Seas, edited by: Herman, Y., Van Nostrand Reinhold Co, New York, 497–569, 1989.
Vilks, G. and Rashid, M. A.: Post-glacial paleo-oceanography of Emerald Basin, Scotian Shelf, Can. J. Earth Sci., 13, 1256–1267, 1976.
Vilks, G., Wagner, F. J. E., and Pelletier, B. R.: The Holocene marine environment of the Beaufort Shelf, Geol. Surv. Can. Bull., 303, 1–43, 1979.
Vilks, G., Deonarine, B., Wagner, F. J., and Winters, G. V.: Foraminifera and Mollusca in surface sediments of southeastern Labrador Shelf, Geol. Soc. Am. Bull., 93, 225–238, 1982.
Weiss, L.: Foraminifera and origin of the Gardiners Clay (Pleistocene), Eastern Long Island, New York, U.S. Geol. Surv. Prof. Paper, 254-G, 139–163, 1954.
Wollenburg, J. E. and Mackensen, A.: Living benthic foraminifera from the central Arctic Ocean: faunal composition, standing stock and diversity, Mar. Micropaleontol., 34, 153–185, 1998.
Østby, K. L. and Nagy, J.: Foraminiferal distribution in the western Barents Sea, Recent and Quaternary, Polar Res., 1, 53–95, 1982.