the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Spine-like structures in Paleogene muricate planktonic foraminifera
Eleanor John
Bridget S. Wade
Simon D'haenens
Caroline H. Lear
Muricate planktonic foraminifera comprise an extinct clade that was diverse and abundant in the Paleogene oceans and are widely used in palaeoclimate research as geochemical proxy carriers for the upper oceans. Their characteristic wall texture has surface projections called “muricae” formed by upward deflection and mounding of successive layers of the test wall. The group is generally considered to have lacked “true spines”: that is, acicular calcite crystals embedded in and projecting from the test surface such as occur in many modern and some Paleogene groups. Here we present evidence from polished sections, surface wall scanning electron microscope images and test dissections, showing that radially orientated crystalline spine-like structures occur in the centre of muricae in various species of Acarinina and Morozovella and projected from the test wall in life. Their morphology and placement in the wall suggest that they evolved independently of true spines. Nevertheless, they may have served a similar range of functions as spines in modern species, including aiding buoyancy and predation and especially harbouring algal photosymbionts, the function for which we suggest they probably first evolved. Our observations strengthen the analogy between Paleogene mixed-layer-dwelling planktonic foraminifera and their modern spinose counterparts.
- Article
(19543 KB) - Full-text XML
- BibTeX
- EndNote
Prominent among Paleogene planktonic foraminifera is an extinct group of “muricate”-walled species (Fig. 1) that are thought to have lacked true spines (Blow, 1979; Benjamini and Reiss, 1979; Hemleben and Olsson, 2006) but nevertheless lived in the upper ocean (Douglas and Savin, 1978; Boersma et al., 1987) and harboured algal photosymbionts like many modern spinose forms (Pearson et al., 1993; D'Hondt et al., 1994; Norris, 1996; Luciani et al., 2017). It is important to understand the ecology of this group because they are frequently used in isotopic and trace element palaeoclimate studies to reconstruct global climate history by recording temporal and spatial changes in upper-ocean conditions, including temperature (e.g. Shackleton and Boersma, 1981; Zachos et al., 1994; Sexton et al., 2006a; Pearson et al., 2007; Aze et al., 2014; Frieling et al., 2017) and pH pCO2 (e.g. Pearson and Palmer, 1999, 2000; Anagnostou et al., 2016, 2020; Henehan et al., 2020). In addition, the carbon isotope (δ13C) proxy has been of particular interest in recent years as a means of understanding carbon and nutrient cycling (John et al., 2013, 2014; Birch et al., 2016) as well as symbiont bleaching events (Wade et al., 2008; Edgar et al., 2013; Luciani et al., 2016, 2017; Shaw et al., 2021). However, questions have been raised as to what extent the unique ecology and separate evolutionary history of the muricate group may have biased their δ13C values (Edgar et al., 2017) and in particular whether the photosymbiont distribution arising from the perceived lack of spines could affect quantitative use of the carbon isotope proxy (Gaskell and Hull, 2019). Here we question the current consensus that the group lacked spines and suggest they were much more similar to modern spinose species than previously thought.
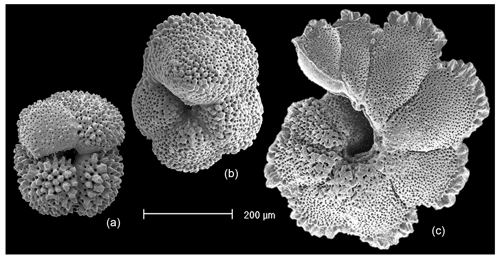
Figure 1Paleogene muricate planktonic foraminifera of the genera Acarinina, Morozovella and Morozovelloides (left to right). In the first two specimens the muricae (surface rugosities) are distributed over most of the test. In Morozovelloides and most species of Morozovella they become fused and concentrated around the periphery and umbilical shoulders of the chambers. (a) Acarinina mcgowrani, outcrop Sample LIN99-17, middle Eocene Zone E13, Kitunda, Tanzania (reproduced from Berggren et al., 2006a); (b) Morozovella subbotinae, Sample TDP14/4/1, 50–60 cm, lowermost Eocene Zones E1–E2; (c) Morozovelloides lehneri, Sample TDP13/8/2, 22–30 cm, middle Eocene Zone E11 (reproduced from Pearson and Berggren, 2006, pl. 10.4, fig. 13).
2.1 Wall layering
Planktonic foraminifera precipitate low-Mg calcite tests composed of successive chambers that are added episodically as the organism grows. Virtually all species have a layered (lamellar) wall texture, although there is currently no agreement as to how the layering is produced. In the classic model of Reiss (1957), each successive chamber initially consists of two layers (lamellae), an inner or “primary” layer that lines the new chamber and an outer or “secondary” layer that coats the new chamber. Usually the primary layer is thinner than the secondary layer. Together, these constitute a “bilamellar unit” (our term), which defines a large group of foraminifera that Reiss (1957) called the Bilamellidae. Subsequent research has shown that the template for calcification is a primary organic sheet (POS) that becomes enclosed between these two layers (Erez, 2003; also referred to as a primary organic membrane, POM, in much of the literature; see Hemleben et al., 1989, and Schiebel and Hemleben, 2017, for details). A key aspect of Reiss's (1957) model is that the secondary layer extends contiguously over the entire external surface of the test, thereby attaching the new chamber and thickening the rest of the test every time a new chamber is added. This explains the common observation that earlier-formed chambers tend to be thicker than later ones. However, an implication of the model is that the final chamber will always consist of two layers (primary and secondary), but this has been questioned by Spero et al. (2015) and Fehrenbacher et al. (2017), who showed that the final chambers of modern species show multiple layers characterized by Mg–Ca banding that are laid down by the adult in a day–night cycle. Clearly these layers are not associated with chamber formation. More research is needed, but one way to harmonize these observations is to extend the model of Reiss to allow additional “adult layers” to be precipitated after the final chamber is formed. Our own observations suggest that adult layers are typically thinner than secondary layers. Finally, at the end of the life cycle, the organism transitions to a new physiological mode called gametogenesis when cellular resources are devoted to the production of large numbers of gametes. Many species show substantial modifications to the test wall at this time, including the shedding or resorption of spines (see below) and the addition of a dense external layer of adult “gametogenic” calcite that may be very thick (Bé, 1980; Hemleben et al., 1989; Eggins et al., 2003). A conceptual scheme for test layering that incorporates primary, secondary, adult and gametogenic layers is shown in Fig. 2.
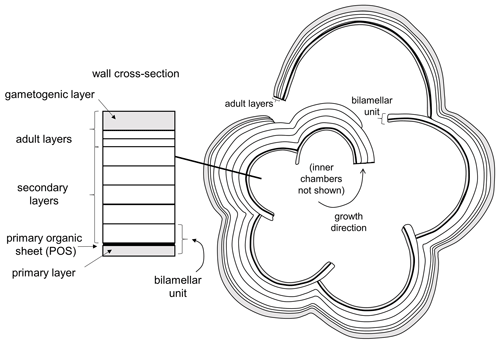
Figure 2Basic pattern of test layering modified from Reiss (1957), Erez (2003) and Fehrenbacher et al. (2017) illustrated in equatorial section. Initial chamber formation consists of a bilamellar unit composed of a primary and secondary layer around the primary organic sheet (POS). The secondary layer covers the entire external surface as each chamber is formed. Additional “adult layers”, including a final gametogenic layer, complete the process.
2.2 Pustules
Planktonic foraminifera of virtually all types can exhibit lumps and spikes on the test wall called “pustules” by Hemleben (1975). These are thought to be primitive features inherited from the earliest species in the Jurassic and serve as anchor points for rhizopods that stream outward from the test surface (Hemleben et al., 1991). They are particularly prevalent along sutures and around the apertures in many species, both spinose and non-spinose, where they presumably have some function regulating the passage of cytoplasm and vacuolated food particles as well as waste materials between the outside and inside of the test. Pustules are generally regarded as being part of the layered wall and can be overgrown by subsequent layers (Hemleben, 1975; Hemleben et al., 1991).
2.3 “True spines”
Early observers of planktonic foraminifera (e.g. Cushman, 1928; Subbotina, 1953) used the terms “spine” and “spinose” to encompass a variety of surface features, but since the 1960s (Parker, 1962) a consensus has emerged that “true” spines are acicular unbranching projections 1–2 µm across “implanted in the wall like a pole” (Hemleben, 1975, p. 335), which emerge orthogonally from the test surface and project outward, often for hundreds of micrometres to millimetres (e.g. Hemleben et al., 1969, 1989; Hemleben, 1975; Schiebel and Hemleben, 2005, 2017; Fig. 3).
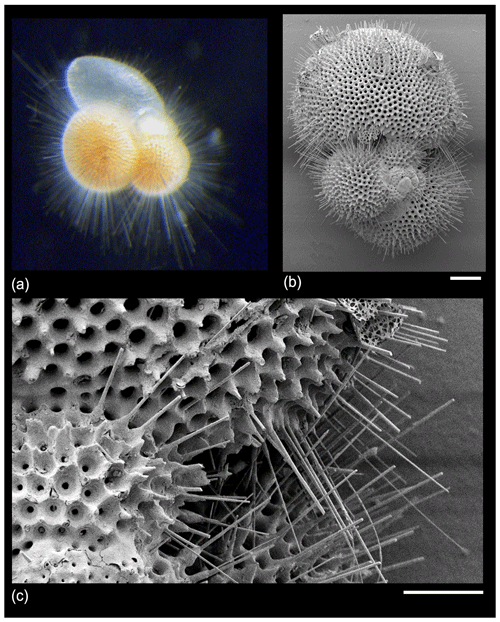
Figure 3“True spines” in Trilobatus sacculifer from the GLOW research cruise, western Indian Ocean offshore of Tanzania. (a) Live specimen photographed shipboard from a plankton tow sample; width of image approximately 1 mm. (b, c) SEM images of a dead pre-gametogenic specimen from a core-top sediment sample. The spine array is broken and would have projected much further from the test in life. Scale bars: (b) 100 µm, (c) 30 µm.
Spines are held to be distinct from other types of ornamentation formed directly on the exterior test surface, which are variously described as pustules (see above), pseudospines, hispidities, muricae, punctae, protuberances, tubercles and ridges (e.g. Hemleben, 1975; Blow, 1979; Benjamini and Reiss, 1979; Hemleben et al., 1991). Spine formation is generally initiated in the early stages of chamber formation at the level of the POS, although reportedly it can also occur on organic sheets at higher levels within the wall (Hemleben et al., 1989, 1991). In our opinion more work is required to establish this and whether the spine rooting position varies between different species. Spines are composed of single crystals of calcite unlike the rest of the test wall, which is made from cemented microgranules (Blow, 1979) or plaques (Hembleben et al., 1989, 1991). Calcification and radial growth occur at the tips of the spines, presumably mediated by the external cytoplasm. The area of test surface around the spines may be strengthened and supported by additional calcite growth, forming “collars”, “bases” or “ridges” (Hemleben, 1975; Hemleben et al., 1991). Successively added test layers plaster neatly around the projecting spines, holding their roots in position. Spines generally form a dense and roughly spherical array around the test, increasing its effective diameter several times over, although in some species they are sparse or concentrated in particular areas such as the chamber tips.
It is important for the organism to coordinate the time and place of gamete release with other members of the species to maximize reproductive success (e.g. Weinkauf et al., 2022). As part of this process, many (not all) species probably sink to a particular depth or density layer at a particular phase of the lunar cycle (Bijma et al., 1990; Schiebel and Hemleben, 2017). Spines tend to be shed or resorbed at the end stage of the life cycle when the cell undergoes gametogenesis, leaving “spine holes” in the wall, which in some species are covered by a final encrustation of gametogenic calcite (Bé, 1980; Hemleben et al., 1989; Caron et al., 1990; Hemleben and Olsson, 2006; Poole and Wade, 2019). The loss of spines reduces drag to enable sinking, and the dense gametogenic calcite layer may also play a role in buoyancy regulation prior to gamete release. A conceptual model for spinose wall textures is shown in Fig. 4.
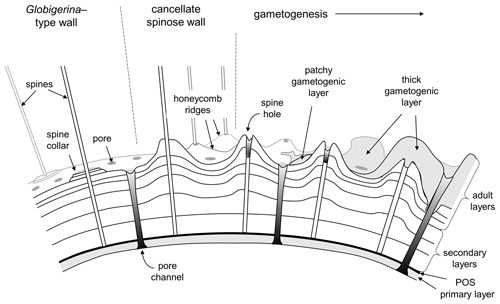
Figure 4Conceptual model of typical spinose wall structures. On the left is a smooth spinose wall with narrow pores and spine collars (e.g. some Globigerina). Centre and right: the more common cancellate spinose arrangement with inter-pore ridges (e.g. Trilobatus). Spines typically project from raised areas on the ridges and at the ridge intersections. Gametogenesis begins with the shedding or resorption of spines, leaving spine holes. A gametogenic layer (shown on the right) is often laid down on top, which can be patchy or overlay the entire test except over the pores. In extreme cases (e.g. Sphaeroidinella) even the pores can be covered over by a cortex.
In the main part of the life cycle prior to gametogenesis, spines serve a variety of functions including supporting external cytoplasm, harbouring photosymbionts, regulating buoyancy and ensnaring metazoan prey (e.g. Spindler et al., 1984; Hemleben et al., 1989; Furbish and Arnold, 1988; Olsson et al., 1999; Gaskell et al., 2019; Grigoratou et al., 2021). By virtue of these adaptations, spinose species are able to occupy niches in the illuminated low-density upper part of the water column in well-stratified and oligotrophic areas, although some specialist species are carnivorous at depth (e.g. Hull et al., 2011). In contrast, non-spinose forms are often herbivorous and tend to live in the more eutrophic areas of the surface ocean in high latitudes or upwelling waters or in deeper thermocline and sub-thermocline habitats where they feed on sinking phytodetritus (e.g. Hemleben et al., 1989; Schiebel and Hemleben, 2017).
Modern planktonic foraminifera divide approximately equally between forms that possess spines (such as Globigerina, Globigerinoides, Orbulina and Trilobatus) and those that do not (such as Globorotalia, Globorotaloides, Pulleniatina and Globigerinita). Genetic evidence (e.g. Darling and Wade, 2008; André et al., 2014) suggests that most or all spinose species are part of a large clade descended from a common ancestor that was presumably itself spinose. The value of spines for higher taxonomy has long been recognized (Parker, 1962; Loeblich and Tappan, 1988) such that spinose species tend to be grouped in superfamily Globigerinoidea, whereas non-spinose species (excepting “microperforate” forms, having pores <1 µm in diameter, which are a separate group) are placed in superfamily Globorotaloidea (Loeblich and Tappan, 1988; Schiebel and Hemleben, 2005, 2017; Brummer and Kučera, 2022). A potential problem with this apparently neat distinction, however, is that spines may have been lost in the evolution of some lineages (e.g. Coxall and Pearson, 2006; Wade et al., 2018b), so not all Globigerinoidea may be spinose. Also, the enigmatic modern genus Hastigerina, which has sparse triradiate spines that have been described as being rooted atop a single wall layer rather than embedded in the wall and therefore do not seem to be homologous with “true” spines, apparently belongs to a separate group (Hemleben et al., 1989; Schiebel and Hemleben, 2017, fig. 6.3).
Tracing spines back through the fossil record is problematic not just because they are generally shed or resorbed late in the life cycle but because they are delicate and vulnerable to diagenetic processes such as dissolution and recrystallization, and they may be abraded in sample processing. The small circular spine holes on the test surface left when spines are shed (and not covered over by late-stage calcification) are more frequently observed in fossils. Hemleben et al. (1991), Liu and Olsson (1994), and Olsson et al. (1992) presented spine-hole evidence that true spines evolved in the very early stages of diversification of the group after the end-Cretaceous mass extinction. According to this model, the first spinose form, Eoglobigerina eobulloides, evolved quickly from a diminutive Cretaceous survivor species of Hedbergella (now Muricohedbergella; Huber and Leckie 2011) and quickly gave rise to a variety of other species within the genera Parasubbotina and Subbotina (see also Koutsoukos, 2014). At the same time, another closely related survivor species of Hedbergella (now Muricohedbergella) gave rise to a parallel radiation of non-spinose forms in the genus Praemurica (Liu and Olsson, 1994; Olsson et al., 1999). Thus, in this model, the phylogenetic divergence of the superfamilies Globigerinoidea and Globorotaloidea dates to the speciation of two species of Muricohedbergella in the latest Cretaceous and the first spines evolved in the immediate aftermath of the mass extinction, presumably because of the extraordinary selection pressures operating at that time.
Subsequent research on Paleogene planktonic foraminifera (Olsson et al., 1999; Pearson et al., 2006; Wade et al., 2018a) has built upon this conceptual framework. True spines (either embedded in the wall or projecting from it in protected areas such as around the aperture) have been observed in a variety of extinct Paleogene forms such as Orbulinoides (Bolli et al., 1957; Premoli Silva et al., 2006), Globoturborotalita (Hemleben and Olsson, 2006; Olsson et al., 2006), Turborotalita (Hemleben and Olsson, 2006; Olsson et al., 2006), Subbotina (Hemleben and Olsson, 2006; Olsson et al., 2006) and Globigerinatheka (Premoli Silva et al., 2006). Spine holes have been observed in all the above genera and some others such as Parasubbotina, Paragloborotalia and Dentoglobigerina (Hemleben et al., 1991; Olsson et al., 1999; Hemleben and Olsson, 2006; Hemleben et al., 2018; Wade et al., 2018b; Fayolle and Wade, 2021). Many of these groups became extinct, however, in the late Eocene and Oligocene, causing a significant constriction in the history of the clade (Ezard et al., 2011; Fraass et al., 2014; Spezzaferri et al., 2015).
2.4 Muricae
A parallel evolutionary radiation is thought to have occurred in Paleogene non-spinose forms stemming from Praemurica (Olsson et al., 1999; Pearson et al., 2006), one part of which gave rise to a large and important clade that includes Acarinina, Morozovella and related genera (family Truncorotaloididae of Loeblich and Tappan, 1961; see also Pearson et al., 2006 and Wade et al., 2018a). These have a distinctive “hispid” appearance (from the Latin “hispidus” for rough or bristly), called “muricate” by Blow (1979), in which the test is covered in small conical or bladed protuberances (see Fig. 1). Although the term muricate is sometimes used to describe some Cretaceous species (e.g. Huber and Leckie, 2011), the typical Paleogene wall texture evolved in the middle part of the Paleocene (Olsson et al., 1999; Birch et al., 2016). Multi-species isotopic and trace element studies have shown that this important group lived in the upper ocean (e.g. Douglas and Savin, 1978; Boersma et al., 1987; Wade, 2004) in association with photosymbionts (Pearson et al., 1993; D'Hondt et al., 1994; Norris, 1996; Wade et al., 2008). They frequently dominate fossil assemblages, particularly in oligotrophic tropical and subtropical locations. Hence, in their life habit they appear to have been analogous to modern spinose groups despite the current consensus (e.g. Hemleben and Olsson, 2006; Hemleben et al., 2018) that they lacked true spines.
In the earlier literature the prominent surface rugosities in this group (muricae of Blow, 1979) were described as “spines” and the texture as “spinose” (e.g. Subbotina, 1953; Bolli, 1957) but only in a general sense. Berggren (1968) and McGowran (1968) suggested that the muricae may have been spine bases or collars with true spines originally projecting outward from them, but subsequent authors rejected that view. In particular, Benjamini and Reiss (1979) and Blow (1979) published independent investigations of the wall texture using scanning electron microscopes (SEMs), including images of deliberately broken specimens in cross-section. Both studies concluded that the structures are unlike true spines and did not act as spine bases. Benjamini and Reiss (1979) drew an analogy with the pustules (surface mounds) of some modern non-spinose species such as Globorotalia truncatulinoides, arguing that once surface rugosities are formed, successive layers of the test wall tend to grow over them, accentuating them and resulting in structures “long enough to be termed `pseudospines”' (Benjamini and Reiss, 1979, p. 143). Blow (1979, pp. 395–401) came to essentially similar conclusions. In 1981, Berggren (1981, p. 103) summarized the new view thus: “neither a central perforation nor distinct rod (spine) run through them. They represent a thickening of the outer lamella and are not true spines, but are, in fact, an inflational ornamentation described often as pustules”. Hemleben and Olsson (2006) produced further images of muricae in cross-section, reiterating that “in contrast to spines they are layered and part of the wall” (Hemleben and Olsson, 2006, pp. 49–50).
This debate leads to the question as to what the distinction between “pustule” and “murica” actually is and whether the latter term is superfluous. We propose that a murica is defined as a type of pustule that involves at least one added layer of calcite contiguous with layering in the primary test wall. As such, muricae probably evolved multiple times, the term being inclusive of layered pustules in modern Globorotalia (Hemleben, 1975; Hemleben et al., 1991) and Cretaceous Muricohedbergella (Huber and Leckie, 2011) and very likely other groups as well, but its most spectacular development was amongst the Paleogene Truncorotaloididae. The “classic” concept of the muricate wall in the Paleogene clade is illustrated in Fig. 5.
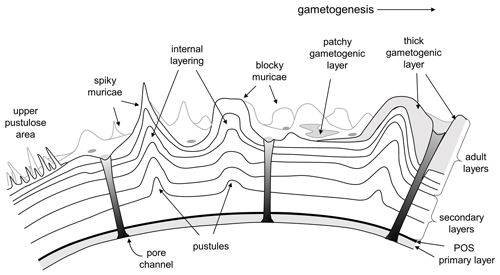
Figure 5Conceptual model of the muricate wall texture in the Paleogene Truncorotaloididae according to the prevailing viewpoint. Muricae are layered protuberances that initiate as small pustules on the external surface of the secondary layer on a newly formed chamber. Successive layers add to the surface rugosity, producing mounded structures with a variety of shapes. Depending on the species, muricae may “fuse” (crowd together) to form structures such as peripheral keels (muricocarinae) or circum-umbilical rings. Gametogenic calcite may be laid down on top, either as thin patches or as extreme thickenings.
Despite the long-standing consensus that muricate planktonic foraminifera were not spinose, there are features of the SEM images of all these previous studies that in our opinion do not sit easily with this interpretation. For instance, Blow (1979) showed several images of muricae with superficial circular holes in the surface revealing empty space beneath. The implication is that successive layers can partly delaminate, leaving a void in the centre of the murical mound that can be revealed by an adventitious hole such as caused (supposedly) by a boring predator (Blow, 1979, pl. 199, figs. 2–4) or surficial abrasion (Blow, 1979, pl. 200, figs. 1–5; pl. 208, figs. 1–4). We note that circular adventitious holes such as those envisaged by Blow (1979) can closely resemble spine holes, and it may not be trivial to distinguish them. Hemleben et al. (1991, p. 121) also noted that spine-hole-like depressions can sometimes be observed on muricae, which they explained as being “due to corrosion (dissolution) of the layering which exposes a cross-section of the tip where the calcite layers are rather thin”. It is generally accepted that successively added test layers do not necessarily broaden and smooth out the growing murica, as would be expected of layers of constant thickness; instead, some muricae evidently become very pointed and sharp (e.g. Benjamini and Reiss, 1979, pl. 1, fig. 9; Blow, 1979, pl. 211, fig. 4; Hemleben and Olsson, 2006, pl. 4.8, fig. 12). This must be caused by successive thinning and steepening of the test layers as added. Hemleben et al. (1991) indicated that newly added layers always completely cover over pre-existing pustules, but some published images seem to show layers that appear to onlap pointed muricae (e.g. Hemleben and Olsson, 2006, pl. 4.8, fig. 12). This can be explained as due to corrosion, but it is not necessarily so. More problematic still is that some published images appear to show a “central object”, typically ∼ 1.5 µm diameter (e.g. Blow, 1979, pl. 208, figs. 1–2; Hemleben and Olsson, 2006, pl. 4.8, figs. 11, 14). These could be interpreted as a tightly constrained peak where the regular chamber wall converges in the centre of the murica and rises sharply upward or as a broken remnant of a distinct rod or spine. We note that cross-sectional images of dissected walls with upward-curving layered muricae lacking a central rod do not disprove the possible existence of such a structure, which would only be revealed if a dissection break runs through the exact centre of the murical mound, the very place where it may be strongest and least likely to fracture.
Thus, the assertion that the muricate wall is non-spinose relies on negative evidence. In this context, it should be stressed that even in Paleogene genera that are widely thought to have been spinose (e.g. Subbotina), spines or spine holes are only rarely encountered and generally only in the best-preserved material and in pre-gametogenetic (thin-walled) specimens. Now new evidence is available in the form of polished specimens embedded in resin imaged in high resolution by SEM, which permits a clear view of the solid test interior. Here we present this evidence alongside a suite of observations of test surfaces and dissections of exceptionally well-preserved specimens from Tanzania with additional material from Integrated Ocean Drilling Program (IODP) Site U1408 (North Atlantic Ocean).
Most of the material examined here is from the Kilwa Group of Tanzania (Nicholas et al., 2006), which is known for the exceptional “glassy” preservation of microfossils such as foraminifera (e.g. Pearson et al., 2001, 2007; Sexton et al., 2006b; Pearson and Wade, 2015) and coccolithophores (Bown et al., 2008; Dunkley Jones et al., 2009). This feature is attributed to the relatively impermeable clay-rich facies (Pearson et al., 2001; Nicholas et al., 2006) and shallow maximum burial depths (van Dongen et al., 2006; Nicholas et al., 2007; Bown et al., 2008), which are thought to have hindered low-temperature recrystallization. This material is from Tanzania Drilling Project (TDP) Sites 2 (Pearson et al., 2004, 2006), 11, 13, 14, 17 and 20 (Nicholas et al., 2006; age model for TDP Site 20 revised in Pearson and Coxall, 2014) and ranges in age from late Paleocene to early Oligocene. Foraminifera were extracted from their clay matrix by soaking in water and gently disaggregating with the fingers before sieving. Individual tests were then picked under a microscope using a paintbrush and mounted on SEM stubs for imaging at the School of Earth and Environmental Sciences at Cardiff University. Additional planktonic foraminifera were selected from middle Eocene (Chron C20n; ∼ 42.5 Ma) drift sediments from IODP Site U1408 (41∘26.30′ N, 49∘47.10′ W; 3022 m water depth, ∼ 2575 m palaeodepth; Norris et al., 2014). Preservation of planktonic foraminifera at this site is generally good but variable owing to the rhythmic lithological alterations of greenish nannofossil-rich clay and whitish nannofossil ooze, in combination with high sedimentation rates of 2–5 cm kyr−1 (Norris et al., 2014; Hull et al., 2017). Samples were dried, disaggregated and (re)washed in deionized water over a 63 µm sieve (Hull et al., 2017). SEM imaging was conducted at the Department of Earth and Planetary Sciences at Yale University. We focused on adult but relatively thin-walled translucent specimens to avoid heavy gametogenic calcite as far as possible.
3.1 Polished sections
Fossil tests of Morozovella crater and M. aragonensis were picked from the >300 µm size fraction of samples from TDP Site 20. The tests were briefly ultrasonicated in methanol and deionized water and embedded in 25 mm blocks of Epothin™2 epoxy resin. The blocks were left to cure at room temperature for at least 7 d, then sanded using fine sandpaper and polished using a 0.3 µm Al2O3 emulsion to expose the cross-section of the test walls. Polished resin blocks were coated with 10–15 nm Ag and examined using an SEM in the Department of Earth Sciences, University of Bristol.
3.2 Surface images and dissections
Specimens were mounted on adhesive carbon discs or double-sided tape atop steel SEM stubs. Some were reserved for direct imaging of the exterior wall, while others were broken open by applying downward pressure with a clean glass slide. Glassy planktonic foraminifera are much stronger than recrystallized specimens and tend to crack into large chunks (Pearson et al., 2015). The resulting pieces were coated with a conductive gold–palladium sputter coat and examined at the School of Earth and Environmental Sciences, Cardiff University, with SEM for indicative features that were then oriented to best effect by tilting and rotating the stage.
4.1 Polished sections
A representative set of SEM micrographs from polished sections is shown in Fig. 6. Figure 6a–c show a specimen of Morozovella crater in side view. Figure 6b shows a slice through the area of thickening around the proloculus on the spiral side. Circular holes are mural pores, which are mostly around 5 µm in diameter. The “bullseye” pattern shows circular layering around “central objects” around 1.5 µm in diameter, which are mostly orthogonal to the plane of section. These objects are clearly integral to the wall structure but do not project outward; they probably originate from an earlier chamber above or below the plane of section. Figure 6c shows cross-sections through several muricae. In these instances the central objects are outwardly directed and seem to begin only on the third test layer from the inside, projecting outward at an angle to the plane of section.
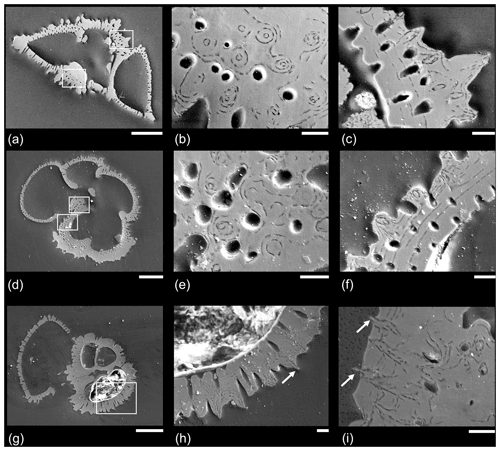
Figure 6Example SEM micrographs of polished specimens of Paleogene muricate planktonic foraminifera. (a–f) Two specimens of Morozovella crater in different orientations, Sample TDP20/32/1, 45–51 cm, middle Eocene Zone E11; (g–h) Acarinina topilensis, Sample TDP20/32/1, 45–51 cm, middle Eocene Zone E11; (i) different specimen of the same species from the same sample, with a close-up of the area on the spiral side. White boxes indicate where close-up images were taken; note that in the case of (e) the areas are approximate because the specimens were polished further to a slightly different level. Arrows indicate instances in which acicular objects project from the test wall. Scale bars: (a, d, g) 100 µm; the rest 10 µm.
Figure 6d–f shows a second specimen of Morozovella crater mounted in umbilical view. Figure 6d illustrates the mode of layering described by Reiss (1957) in which a distinct layer is added over the entire test every time a chamber is formed; hence, the final chamber (to the left of the image) is the thinnest. Nevertheless, it consists of at least four layers, showing that secondary calcite has been added in maturity after the final chamber was formed (see Sect. 2.1 above for discussion). Figure 6e is from a slice through the earliest chamber in the final whorl in the area where the test is thickest. The image shows a similar bullseye effect as Fig. 6b, but note that the circular features at the centre of each concentric pattern tend to be wider towards the test interior. Figure 6f is from part of the same chamber beneath the aperture, showing a series of muricae and mural pores that cross the wall at an oblique angle to the plane of section. At least nine layers are visible, becoming thinner as added. There are more layers than chambers in the final whorl; hence, not all the layering is associated with chamber formation. These “adult layers” (see Fig. 2), especially, appear to bend upward sharply and wrap around a central object that is about 1 µm in diameter.
Figure 6h, from a specimen of Acarinina topilensis, shows cross-sections through several muricae including one for which there is evidence (arrow) that a central object projects beyond the test wall. Figure 6i, from the spiral side of another specimen of the same species, shows that the final three layers appear to flatten out the pre-existing rugosities by preferentially filling in the spaces between them. Nevertheless, the delicate central structures project a short distance beyond the wall surface (arrows) to a point at which they were probably broken off in the sediment or during sample processing.
The external appearance of two specimens of Morozovella subbotinae from the earliest Eocene is shown in Fig. 7. Both specimens have a partial gametogenic layer, which is especially pronounced in areas of the keel (“muricocarina” of Blow, 1979) and umbilical shoulders of the chambers. Some of the muricae are covered by a smooth layer of gametogenic calcite, which is highlighted in Fig. 7b. Note that the surface here is unlikely to be abraded because it is protected by the overhang of the adjacent (penultimate) chamber. In the centre of every exposed murica is a central object with the appearance of a vertically oriented solid rod ∼ 2 µm in diameter wrapped around by concentric layers that feature the microgranular texture typical of the normal test wall. These rods tend to terminate in oblique fracture surfaces consistent with the rhombohedral cleavage angles of calcite oriented with a vertical c axis. We suggest that these images represent the typical state of muricae during gametogenesis before the calcite crust is plastered over the top of them. Similar features are shown by the second specimen and were observed on several other individuals of this species examined, as well as other Morozovella and Acarinina (not shown), except that the central objects range in diameter from ∼ 1 to 3 µm depending on the species and individual.
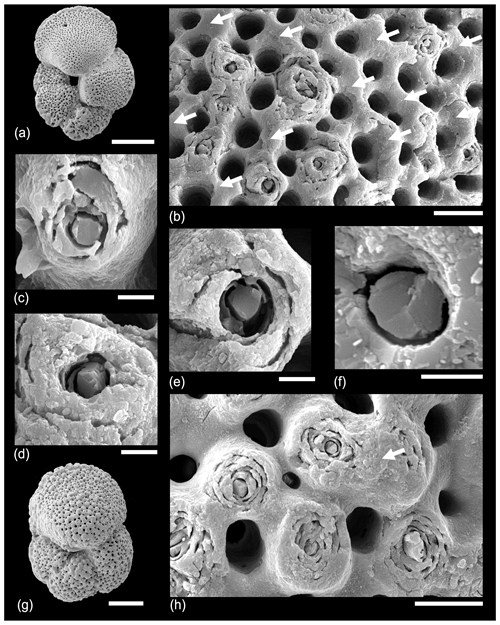
Figure 7SEM micrographs of Morozovella subbotinae, Sample TDP14/14/2, 10–20 cm (lowermost Eocene Zones E1–E2). (a) General view of test with partial gametogenic crust. (b) Detail of a wall about half-covered in gametogenic calcite (arrows), which covers some muricae but not others. (c, d, e, f) Details of muricae showing crystalline central object broken cleavage planes. (g, h) A second specimen. (h) A murica partially covered with gametogenic calcite highlighted. Scale bars: (a, g) 100 µm, (b, h) 10 µm; the rest 2 µm.
In Fig. 8, various specimens of Acarinina from a range of ages from Tanzania and IODP Site U1408 are shown. Figure 8a–b shows a specimen of Acarinina soldadoensis with a heavy calcite crust but also featuring a “hollow” murica that looks very like a spine hole. This is similar to some of Blow's (1979) original images of hollow muricae, but we suggest it is caused by the shedding of the central object prior to gametogenesis rather than predation. Figure 8c–e show Acarinina rohri from the middle Eocene, contrasting a specimen showing exposed muricae (Fig. 8d) with a heavily calcified test in which the muricae are almost all covered by gametogenic calcite (Fig. 8e). Figure 8f–i show a moderately well-preserved specimen of Acarinina puntocarinata from IODP Site U1408, Newfoundland Drift, North Atlantic Ocean. Figure 8g shows an abraded area around the umbilical shoulder of the first chamber in the final whorl, revealing the internal structure of the muricae. Figure 8h shows a typical area of the final chamber with a thin gametogenic layer and conical muricae. Figure 8f–h show one of the last surviving muricate species, Acarinina collactea, from the lower Oligocene of Tanzania, demonstrating continuity of wall texture throughout almost the entire time the group persisted.
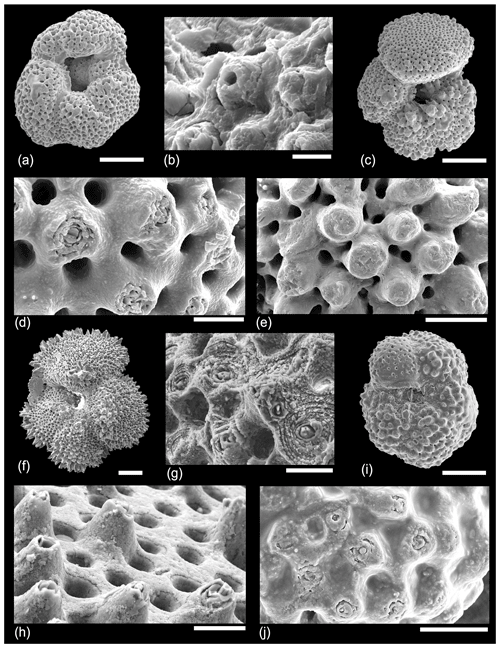
Figure 8SEM micrographs of various species showing details of the external wall structure. (a–b) Acarinina soldadoensis, Sample TDP14/14/2, 10–20 cm (lowermost Eocene Zones E1–E2). (b) Murica with central hole; (c, e) Acarinina rohri, Sample TDP13/13/1, 21–36 cm (middle Eocene Zone E11); (d) detail of another specimen from the same sample showing exposed or abraded murica; (e) detail of final chamber showing muricae covered by gametogenic calcite; (f–h) Acarinina punctocarinata, Sample U1408/B/14H/3, 31–33 cm, Sample 342-U1408B-14H-3, 31–33 cm (middle Eocene Zones E10–E11); (i, j) Acarinina collactea, Sample TDP11/19/1, 60–75 cm (lower Oligocene Zone O1) (reproduced from Pearson and Wade, 2015, fig. 30.1a–b). Scale bars: (a, d, f) 100 µm, (b, h) 10 µm, (c, e, g) 5 µm.
4.2 Test dissections
In an attempt to understand the structure of the muricae in cross-section we dissected a number of specimens of Morozovella, Acarinina and Morozovelloides from different stratigraphic intervals. A selection of images are illustrated in Fig. 9. Figure 9a shows a typical area of test wall, which we interpret as a mature but pre-gametogenic state. The mural pores are lined by an organic layer, and remnants of the organic “pore plug” (Hemleben et al., 1989) may also be visible. Muricae seem to initiate above the secondary layer in most instances and show glimpses of a central structure in some places. Figure 9b is an off-centre cut showing how the murica is built by upward deflection of the chamber wall. The microgranular wall texture confirms that everything in this image is part of the primary test wall. Figure 9c shows an oblique view of a broken murica looking down from above. The central structure apparently initiates at the second layer in this instance and is partly coated in thin upraised layers of test wall. Figure 9d shows a central object, broken off in the lower part, projecting through a murica with wall layers that steepen and lap onto it. Figure 9e shows a broken murica with a vertically oriented central object. Figure 9f shows a very interesting image (unique in our investigation) of a murica with a central object that appears to terminate in a trigonal point reminiscent of an inorganic “nailhead spar”. The object itself may have crystal faces along its length, a feature seen in some of the external images (e.g. Fig. 7d, e). Various thin test layers onlap the object as is normal, but it is overlain by a thick calcite crust. We suggest this represents the normal situation in post-gametogenic individuals at the end of the life cycle. Figure 9g is another revealing image that appears to show a central object running orthogonally through the test wall, which has shattered along cleavage planes upon breakage. The rhombohedral cleavage intersections are typical of calcite. In this instance the primary test wall is platy rather than microgranular as occurs in some species. The final image, Fig. 9h, from the same specimen as Fig. 9g, shows a murica as viewed obliquely upward from the interior. The central object shows distinct crystal faces or cleavage planes. The image also illustrates another phenomenon we have noticed in various instances, namely that the crystalline nature of the structure seems to have encouraged the first stages of diagenetic recrystallization in its immediate vicinity as the adjacent layers also appear crystalline.
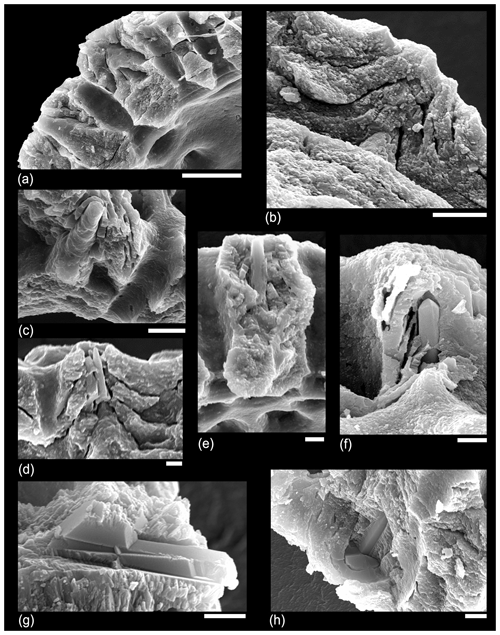
Figure 9SEM micrographs of various dissected specimens of Morozovella and Acarinina illustrating the internal structure of muricae. (a) Morozovella subbotinae, Sample TDP14/4/2, 10–20 cm (lowermost Eocene Zones E1–E2). (b) Morozovella subbotinae, Sample TDP14/8/2, 86–96 cm (lowermost Eocene Zones E1–E2). (c) Morozovella subbotinae, Sample TDP14/4/2, 10–20 cm (lowermost Eocene Zones E1–E2). (d) Acarinina soldadoensis, Sample TDP14/4/2, 10–20 cm (lowermost Eocene Zones E1–E2). (e) Acarinina bullbrooki, TDP13/13/1, 21–36 cm; (f–h) (middle Eocene Zone E11), Morozovella aequa, Sample TDP14/4/2, 10–20 cm (lowermost Eocene Zones E1–E2). Scale bars: (a) 10 µm; (b, c) 5 µm; (d, e, f, g, h) 2 µm.
As mentioned in Sect. 2.2 above, many species of planktonic foraminifer, both spinose and non-spinose, develop a distinctly pustulose surface texture, especially around the aperture and along the sutures. In many of our dissections we have observed clusters of spiky pustules, which grow upward from the primary test wall in these areas (Fig. 10). It is important to distinguish such unlayered pustules from muricae, although they may bear a relation to one another in terms of calcification mechanism. Figure 10a shows a wall cross-section (partly obscured by debris in places) with internal layering rising toward thick bladed muricae on which a thin pustulose layer is developed. Figure 10b illustrates that the pustulose layer is superficial. Figure 10c and d show how the central object within muricae may resemble pustules in size and shape and project upward through the pustulose layer. Figure 10e–g show a broken specimen of Morozovelloides successively zoomed in to show a densely pustulose area that was previously in the aperture at the base of the final chamber, which has now broken off. This texture is similar to the extreme development of spiky pustules (“barbules”) illustrated by Pearson and Wade (2015) in Globoturborotalita barbula, a species that also has true spines, which project through the pustulose layer. We have observed identical structures from IODP Site U1408 (not shown).
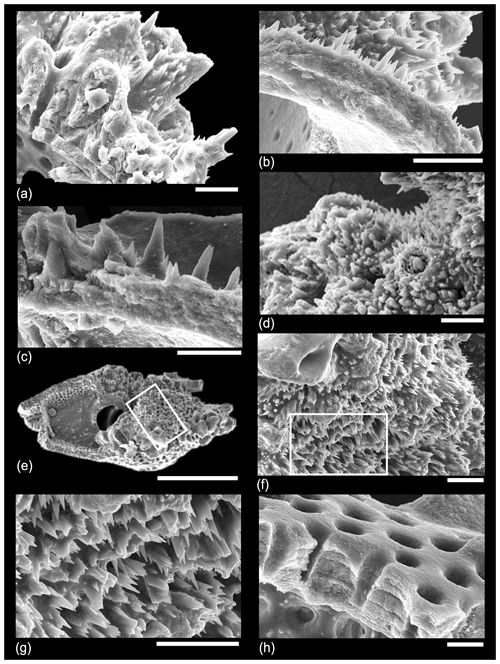
Figure 10SEM micrographs of various dissected muricate species illustrating pustulose and smooth areas of the wall. (a) Morozovella acuta, Sample TDP14/4/2, 10–20 cm (lowermost Eocene Zones E1–E2); (b) another specimen from the same sample; (c) Morozovella occlusa, Sample TDP14/4/2, 10–20 cm (lowermost Eocene Zones E1–E2); (d) Acarinina rohri, Sample TDP13/13/1, 21–36 cm (middle Eocene Zone E11); (e–g) Morozovelloides lehneri, Sample TDP13/13/1, 21–36 cm (middle Eocene Zone E11); (h) another specimen from the same sample showing an area of smooth wall with no muricae. Scale bars: all 10 µm except for (e), which is 100 µm.
While most Acarinina species typically have muricae distributed evenly over the entire test, there is a repeated tendency for more specialized forms to evolve in which muricae are concentrated in particular areas, especially around the sutures, umbilicus and periphery. Concentrations of fused bladed muricae can produce a ragged keel-like structure called a muricocarina by Blow (1979). We crushed 10 well-preserved specimens of Morozovelloides from middle Eocene Zone E11 of TDP Site 13. Despite careful observation of the chamber surfaces, we did not observe the characteristic features of muricae, nor did we observe any bullseye features or central objects. We suggest that the Morozovelloides lineage responded to a selection pressure that favoured a discoidal arrangement of cytoplasm and lost most of its muricae in the process.
5.1 Interpretation and terminology
Were Paleogene muricate planktonic foraminifera spinose? This question requires a coherent interpretation of the various features observed and a reconsideration of the terms “spine”, “pustule” and “murica” as applied to the group. Like the true spines of the Globigerinoidea, the spacing of the central objects within the muricae is fairly regular and respects the position of the mural pores. However, they seem to differ from true spines in important respects. The bases appear to be conical, very like the pustules that have been observed in many species including non-spinose forms such as modern Globorotalia (e.g. Hemleben, 1975). The muricae are then formed by layering of the test wall over and around this central structure. The objects seem more variable in diameter than true spines, not just near their flaring bases but between species. They may have formed less dense arrays than occur in many species of Globigerinoidea (e.g. Fig. 2). Most importantly, they do not appear to originate at the POS but always atop secondary layers and apparently at different levels in the wall. Thus, the muricate clade appears not to be part of superfamily Globigerinoidea, and there is no reason to upset the widely accepted phylogenetic history of the group (Olsson et al., 1999; Pearson et al., 2006).
Although the central objects of muricae may be highly modified pustules, we differ from the current view in two main respects. Firstly, they are clearly crystalline with radially oriented c axes, which means they are not “layered like the test wall itself” (Hemleben, 1975, p. 336) but must have been produced by a calcification mechanism different from that which produced the test wall. Secondly, the central objects clearly project through and above the regular microgranular layers of the test, which onlap onto them (e.g. Fig. 9d). In life the objects extended through the outer layers up to the test surface, and in some instances we have observed them extending above it (Fig. 6h, i). There is no reason to think they could not have projected great distances in the manner of true spines. Indeed, given the highly specialized and evolved nature of the central objects, their crystalline structure which provides a template for upward growth, their stoutness at the surface, and the manifest adaptive advantages they would have conveyed by projecting outwards, it seems likely that they did exactly that. This has not yet been observed because long acicular projections would be unlikely to survive standard sample processing. Direct SEM observation of mudrock fracture surfaces (e.g. Bown et al., 2008) is a possible line of future research that might produce such evidence.
The analogy between the central objects and true spines is strengthened further by the fact that if the former did indeed project outward, they were evidently shed at gametogenesis, producing spine holes. We have observed multiple instances in which gametogenic calcite overlays the central objects either in exterior view or cross-section (e.g. Figs. 7h, 9f). Thus, we can infer that like true spines, the objects were important for the main life functions of the organisms (such as feeding and symbiosis) but became unwanted at the final gametogenic phase. As discussed above (Sect. 2.1), at this time in the life cycle, gamete production and buoyancy regulation become crucial so as to coordinate gamete release with other members of the species by sinking to a particular depth and/or water density. In spinose species, the external drag caused by spines hinders this function, which is probably why they are shed. The same was likely the case for the central objects in muricate species.
Blow's (1979) term “murica” is derived from the Latin murus (wall) to emphasize his contention that the mounds were formed by layering of the primary test wall and were not separate objects added onto the wall. This term is accurate for the layered mounds that form the bulk of these structures, and the descriptive term “muricate” remains appropriate for the group as a whole and their distinctive wall texture. The term does not, however, encompass the central objects observed in this study, which are crystalline and not part of the main wall. Given the fact that orthogonal “spines” are already thought to have evolved twice in the Cenozoic planktonic foraminifera (in the Globigerinoidea and Hastigerina group; see Schiebel and Hemleben, 2017) we suggest that the common English language word spine, as used widely across zoology and botany for convergent features, is appropriate to describe the central objects and that the muricates provide a third (and very successful) example of spinose planktonic foraminifera. In Fig. 11 we illustrate this interpretation of the typical muricate wall both before and after gametogenesis.
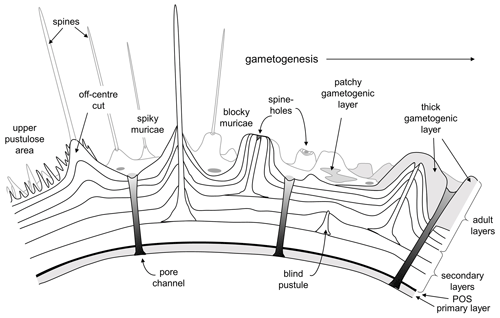
Figure 11New interpretative representation of the muricate wall in cross-section based on our findings. Note that the spines initiate as pustules on the external surface of various secondary layers and not at the primary organic sheet (POS). “Blind pustules” are where pustules do not extend into spines running all the way to the test surface. Areas of the adult test, especially around the aperture, may also be covered in very fine spiky pustules.
5.2 Evolution
An interesting aspect of the fossil record is that isotopic evidence indicates that none of the earliest Paleocene species lived in association with symbionts, and, by implication, they may not have been able to fully exploit surface ocean oligotrophic habitats (Norris, 1996; Coxall et al., 2006; Birch et al., 2016). This includes the early members of the Globigerinoidea with true spines. According to Blow (1979) and Olsson et al. (1999), the muricate wall texture evolved in the mid-Paleocene in the transition from Praemurica uncinata (first appearance 61.4 Ma on the timescale of Cande and Kent, 1995; see Wade et al., 2011) to Morozovella praeangulata (first appearance 61.2 Ma), which within ∼ 200 kyr gave rise to three major clades via Morozovella angulata, Igorina pusilla and Acarinina strabocella. Isotopic work suggests that P. uncinata was the first Paleogene species to evolve a photosymbiotic relationship with algae (Norris, 1996; Coxall et al., 2006; Birch et al., 2016). Clearly it would be very interesting to examine the evolution of the muricate wall in well-preserved Paleocene material to investigate to what extent it was gradual or sudden and whether the evolution of spines in the group was directly coincident with the evolution of photosymbiosis as evidenced by test chemistry. It seems highly likely that a novel spine–symbiont association was the key innovation that allowed the group to exploit new niches and caused the adaptive radiation.
Despite having evolved spines before the muricate group, most Globigerinoidea in the Paleogene (e.g. Subbotina spp.) seem to have continued living deeper in the water column without photosymbionts (e.g. Boersma et al., 1987; Pearson et al., 1993; Norris, 1996). There are, however, exceptions, most notably Eocene Globoturborotalita and Globigerinatheka, which had true spines and moved into surface habitats, probably with photosymbionts (Premoli Silva et al., 2006; Sexton et al., 2006a; Edgar et al., 2013). Nevertheless, the muricate clade may have benefitted from an incumbency advantage in shallow-water oligotrophic environments for much of its history. The great radiation of modern spinose species only occurred after the extinction of the muricates (Aze et al., 2011; Fraass et al., 2014). Therefore, the presence of true spines in the Globigerinoidea appears to be a case of “pre-adaptation” to a photosymbiotic life habitat.
The muricate clade diversified into a number of genera, reaching maximum diversity in the early to middle Eocene (Aze et al., 2011; Fraass et al., 2014). While the ancestral condition was a wall texture evenly covered by muricae, there was an iterative tendency to evolve concentrations of densely packed muricae around the test periphery in a keel-like structure that Blow (1979) called a muricocarina, as well as along the sutures and on the umbilical shoulders of the chambers in the final whorl. The evolution of muricocarinae seems to have occurred at least three times, leading to the genera Morozovella (Olsson et al., 1999), Planorotalites (Berggren et al., 2006b) and Morozovelloides (Pearson and Berggren 2006). In each of these groups there are species which also show a marked reduction in muricae in other areas of the chamber surfaces, which become smooth and shiny. An extreme example of this is Morozovelloides lehneri (see Fig. 1), which has a large discoidal morphology with muricae only around the umbilicus and the periphery. The resulting morphology is reminiscent of some discoidal non-spinose Neogene Globorotalia, but stable isotope studies indicate that M. lehneri and the other specialized muricates retained a shallow mixed-layer symbiotic habit (Boersma et al., 1987; Pearson et al., 2001; Pearson, 2012; Edgar et al., 2015; Anagnostou et al., 2016).
The muricate group suffered a major extinction shortly before the end of the middle Eocene, at which time Morozovelloides and large Acarinina became extinct in quick succession (Wade, 2004; Wade et al., 2012). Small acarininids with the characteristic spinose muricate wall survived into the late Eocene and early Oligocene, but only at low diversity and abundance (Wade and Hernitz Kucenjak, 2018; Wade et al., 2021). The “modern”-style taxonomic and ecological order of planktonic foraminiferal assemblages developed throughout the Miocene as the spinose Globigerinoidea refilled many of the ecological niches left vacant by the muricates, albeit with a substantial intervening time gap.
If acicular spines have evolved repeatedly, it follows that their presence or absence is of less fundamental importance for the taxonomy of the group than had generally been assumed: it is the particular type of spine that is important. As discussed above, spines appear to have been lost in the evolution of some groups. There may have been other occasions when they evolved independently or perhaps re-evolved using common genetic pathways, although that has yet to be demonstrated. Our observations support the broader contention that because wall textures have evolved significantly, future phylogenetic work should allow for that as part of a total evidence approach that integrates information from morphological traits, genetics and stratigraphy.
5.3 Carbon metabolism
One area in which the issue of spines in the muricates may be significant for palaeoclimate research relates to reconstructions of ambient seawater carbon isotope (δ13C) values and gradients. The relatively high δ13C values of modern spinose species relative to other groups is widely thought to be a function of their surface habitat (δ13C tends to be higher in the upper water column because of the biological pump) and photosymbiotic association (symbionts preferentially remove the light isotope 12C from around the test) (see Birch et al., 2013, for a discussion of these effects). Nevertheless, there appears to be a limited size range in which the photosymbiont vital effect is counteracted by other effects such as the incorporation of respiratory carbon so that the test δ13C is close to the ambient environmental δ13C of dissolved inorganic carbon (Birch et al., 2013). Thus, data from this size range can be used alongside deeper-dwelling species to approximate the δ13C gradient through the upper water column (Birch et al., 2013). A similar approach was taken by John et al. (2013, 2014) for the Paleogene oceans based on the assumption that the same factors that influence the δ13C of Neogene spinose species also apply in the Paleogene muricates. But Gaskell and Hull (2019) questioned this assumption on the grounds that the muricates may have had a radically different photosymbiont arrangement and carbon metabolism. They suggested that a lack of spines would result in a mat-like arrangement of symbionts close to the test surface, which would necessarily produce a stronger local vital effect fractionation than occurs in Neogene spinose species. Consequently, they argued, global patterns of temperature-dependent carbon export reconstructed using the Paleogene muricates (e.g. John et al., 2013, 2014) may be artificially exaggerated. Our new observation that the muricates were in fact spinose obviates this as a general criticism. We also note that the temperature-dependent relationship suggested for the Paleogene oceans is consistent with recent Neogene warm-period data using species with true spines (Boscolo-Galazzo et al., 2021). This does not mean, however, that all species of the Globigerinoidea or muricate clades can necessarily be assumed to have an identical symbiont distribution and carbon metabolism; there is plenty of scope for species-specific variability in both clades throughout the evolutionary history of the group, which still needs to be teased out.
The analogy between Neogene and Paleogene spinose groups is not just important for carbon isotope studies. Photosymbionts have a marked effect on the pH of the microenvironment they inhabit, which is directly relevant for the boron isotope (δ11B) proxy. Quantitative interpretation of δ11B as both a pH and pCO2 proxy requires explicit assumptions relating to species-specific fractionations and mode of life (e.g. Anagnostou et al., 2015). If Paleogene muricate and spinose photosymbiotic species generally fractionated boron in a similar way as Neogene symbiotic species with true spines, then long-term trends in global pCO2 will be easier to reconstruct with confidence and more quantitative reliability.
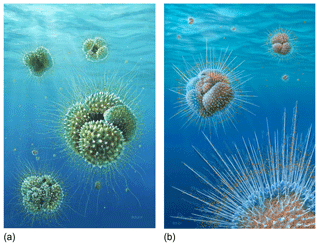
Figure 12Reconstructions of Acarinina mcgowrani in life showing two models for the wall texture and symbiont ecology, painted by Richard Bizley with scientific input by PNP. (a) The previously prevailing view of muricae, painted in 2011, showing symbionts in a mat-like arrangement close to the test surface. (b) Revised view, painted for this study. Muricae support an array of stout spines with symbionts able to deploy away from the test surface as in modern spinose planktonic foraminifera. Image copyright Richard Bizley (https://bizleyart.com, last access: 22 July 2022).
We have used observations from polished sections and external and dissected test surfaces to suggest that the Paleogene muricate planktonic foraminifera were spinose (Fig. 12). The old view that muricae (mounds on the surface) are spine bases (Berggren, 1968; McGowran, 1968) seems correct in retrospect. However, the spines are not homologous with the “true” spines of the diverse modern clade Globigerinoidea, principally differing in that they initiate on secondary layers within the test wall rather than on the primary organic sheet. The evolution of muricae was closely associated with the development of a photosymbiotic ecology. The separate evolution of spines in the Globigerinoidea and the muricate clade is a striking instance of iterative evolution. The Paleogene muricates were much more similar in morphology, ecology and probably test geochemistry to their modern spinose counterparts (e.g. Globigerinoides and Trilobatus) than previously thought, which has implications for the interpretation of a variety of palaeoceanographic proxies, particularly those based on carbon and boron isotopes.
No data sets were used in this article.
Observations were made by PNP, EJ (polished specimens), BSW (TDP Site 11) and SD (IODP Site U1408). PNP prepared the paper with contributions from all authors.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Funding was provided by NERC grant NE/P019102/1 to Caroline H. Lear and Paul N. Pearson as part of the SWEET (Super-Warm Early Eocene Temperatures and climate) consortium (https://www.deepmip.org/sweet/, last access: 22 July 2022). We are grateful to Chris Poole for Fig. 3b and c. We thank Dan Lunt (University of Bristol) for coordinating that project and funding the artwork in Fig. 12. The Belgian American Educational Foundation and the Fulbright Commission of Belgium and Luxemburg provided support to Simon D'haenens. Tanzanian foraminifera were previously obtained with NERC grants GR3/12618, NE/X509345/1 and NE/B503225/1 to Paul N. Pearson. We thank the Tanzania Commission of Science and Technology (Costech) and Tanzania Petroleum Development Corporation (TPDC) for assistance in that research and the various members of the Tanzania Drilling Project field teams, especially Joyce Singano of TPDC. We thank Brian Huber, Michal Kucera and an anonymous reviewer for their constructive comments in review.
This research has been supported by the Natural Environment Research Council (grant nos. NE/P019102/1, GR3/12618, NE/X509345/1, and NE/B503225/1).
This paper was edited by Francesca Sangiorgi and reviewed by Brian Huber, Michal Kucera, and one anonymous referee.
Anagnostou, E., John, E. H., Edgar, K. M., Foster, G. L., Ridgwell, A., Inglis, G. N., Pancost, R. D., Lunt, D. J., and Pearson, P. N.: Atmospheric CO2 concentration was the primary driver of early Cenozoic climate, Nature, 533, 380–384, https://doi.org/10.1038/nature17423, 2016.
Anagnostou, E., John, E. H., Babila, T. L., Sexton, P. F., Ridgwell, A., Lunt, D. J., Pearson, P. N., Chalk, T. B., Pancost, R. D., and Foster, G. L.: Proxy evidence for state-dependence of climate sensitivity in the Eocene greenhouse, Nat. Commun., 11, 1–9, https://doi.org/10.1038/s41467-020-17887-x, 2020.
André, A., Quillévéré, F., Morard, R., Ujiié, Y., Escarguel, G., de Vargas, C., de Garidel-Thoron, T., Douady, C. J., and Ketmaier, V.: SSU rDNA divergence in planktonic Foraminifera: molecular taxonomy and biogeographic implications, PLoS ONE, 2014, 0104641, https://doi.org/10.1371/journal.pone.0104641, 2014.
Aze, T., Ezard, T. H. G., Purvis, A., Coxall, H. K., Stewart, D. R. M., Wade, B. S., and Pearson, P. N.: A phylogeny of Cenozoic macroperforate planktonic foraminifera from fossil data, Biol. Rev., 86, 900–927, https://doi.org/10.1111/j.1469-185X.2011.00178.x, 2011.
Aze, T., Pearson, P. N., Dickson, A. J., Badger, M. P. S., Bown, P. R., Pancost, R. D., Gibbs, S. J., Huber, B. T., Leng, M. J., Coe, A. S., Cohen, A. S., and Foster, G. L.: Extreme warming of tropical waters during the Paleocene-Eocene Thermal Maximum, Geology, 42, 739–742, https://doi.org/10.1130/G35637.1, 2014.
Bé, A. W. H.: Gametogenic calcification in a spinose planktonic foraminifer, Globigerinoides sacculifer (Brady), Marine Micropaleontol., 5, 283–310, 1980.
Benjamini, C. and Reiss, Z.: Wall-hispidity and -perforation in Eocene planktonic foraminifera, Micropaleontology, 25, 141–150, 1979.
Berggren, W. A.: Phylogenetic and taxonomic problems of some Tertiary planktonic foraminiferal lineages, Tulane Stud. Geol. Paleont., 6, 1–22, 1968.
Berggren, W. A.: Review of Blow, Walter H., The Cainozoic Globigerinida: A study of the morphology, taxonomy, evolutionary relationships and the stratigraphical distribution of some Globigerinida (mainly Globigerinacea), Micropaleontology, 27, 99–108, 1981.
Berggren, W. A., Pearson, P. N., Huber, B. T., and Wade, B. S.: Taxonomy, biostratigraphy and phylogeny of Eocene Acarinina, in: Atlas of Eocene Planktonic Foraminifera, edited by: Pearson, P. N., Olsson, R. K., Huber, B. T., Hemleben, C., and Berggren, W. A., Cushman Foundation, Special Publication 41, 257–326, 2006a.
Berggren, W. A., Olsson, R. K., and Premoli Silva, I.: Taxonomy, biostratigraphy and phylogenetic affinities of Eocene Astrorotalia, Igorina, Planorotalites, and Problematica (Praemurica? lozanoi), in: Atlas of Eocene Planktonic Foraminifera, edited by: Pearson, P. N., Olsson, R. K., Huber, B. T., Hemleben, C., and Berggren, W. A., Cushman Foundation, Special Publication 41, 377–400, 2006b.
Bijma, J., Erez, J., and Hemleben, C.: Lunar and semilunar reproductive cycles in some spinose planktonic foraminifers, J. Foramin. Res., 20, 117–127, https://doi.org/10.2113/gsjfr.20.2.117, 1990.
Birch, H., Coxall, H. K., Pearson, P. N., Kroon, D., and O'Regan, M.: Planktonic foraminifera stable isotopes and water column structure: Disentangling ecological signals, Mar. Micropaleontol., 101, 127–145, https://doi.org/10.1016/j.marmicro.2013.02.002, 2013.
Birch, H., Coxall, H. K., Pearson, P. N., Kroon, D., and Schmidt, D.: Partial collapse of the marine carbon pump after the Cretaceous-Paleogene boundary, Geology, 44, 287–290, https://doi.org/10.1130/G37581.1, 2016.
Blow, W. H.: The Cainozoic Globigerinida, E.J. Brill, Leiden, 3 Volumes, 1413 pp., 1979.
Boersma, A., Premoli Silva, I., and Shackleton, N. J.: Atlantic Eocene planktonic foraminiferal paleohydrographic indicators and stable isotope paleoceanography, Paleoceanography, 2, 287–331, 1987.
Bolli, H. M.: Planktonic foraminifera from the Eocene Navet and San Fernando formations of Trinidad, B.W.I, United States National Museum Bulletin, 215, 155–172, 1957.
Boscolo-Galazzo, F., Crichton, K. A., Ridgwell, A., Mawbey, E. N., Wade, B. S., and Pearson, P. N.: Temperature controls carbon cycling and biological evolution in the ocean twilight zone, Science, 371, 1148–1152, https://doi.org/10.1126/science.abb6643, 2021.
Bown, P. R., Dunkley Jones, T., Lees, J. A., Randell, R. D., Mizzi, J. A., Pearson, P. N., Coxall, H. K., Young, J. R., Nicholas, C. J., Karega, A., Singano, J., and Wade, B. S.: A Paleogene calcareous microfossil Konservat-Lagerstätte from the Kilwa Group of coastal Tanzania, Geol. Soc. Am. Bull., 120, 3–12, https://doi.org/10.1130/B26261.1, 2008.
Brummer, G.-J. and Kučera, M.: Taxonomic review of living planktonic foraminifera, J. Micropalaeontol., 41, 29–74, https://doi.org/10.5194/jm-41-29-2022, 2022.
Cande, S. C. and Kent, D. V.: Revised calibration of the geomagnetic polarity timescale for the Late Cretaceous and Cenozoic, J. Geophys. Res.-Solid, 100, 6093–6095, https://doi.org/10.1029/94JB03098, 1995.
Caron, D. A., Anderson, O. R., Lindsey, J. L., Faber Jr., W. W., and Lim, E. L.: Effects of gametogenesis on test structure and dissolution of some spinose planktonic foraminifera and implications for test preservation, Mar. Micropaleontol., 16, 93–116, 1990.
Coxall, H. K. and Pearson, P. N.: Taxonomy, biostratigraphy, and phylogeny of the Hantkeninidae (Clavigerinella, Hantkenina, and Cribrohantkenina), in: Atlas of Eocene Planktonic Foraminifera, edited by: Pearson, P. N., Olsson, R. K., Huber, B. T., Hemleben, C., and Berggren, W. A., Cushman Foundation, Special Publication 41, 216–225, 2006.
Coxall, H. K., D'Hondt, S., and Zachos, J. C.: Pelagic evolution and environmental recovery after the Cretaceous-Paleogene mass extinction, Geology, 34, 297–300, https://doi.org/10.1130/G21702.1, 2006.
Cushman, J. A.: Foraminifera their classification and economic use, Cushman Lab Foram. Res. Spec. Publ., 1, 1–401, 1928.
Darling, K. F. and Wade, C. M.: The genetic diversity of planktic foraminifera and the global distribution of ribosomal RNA genotypes, Mar. Micropaleontol., 67, 216–238, https://doi.org/10.1016/j.marmicro.2008.01.009, 2008.
D'Hondt, S., Zachos, J. C., and Schultz, G.: Stable isotope signals and photosymbiosis in late Paleocene planktic foraminifera, Paleobiology, 20, 391–406, 1994.
Douglas, R. G. and Savin, S. M.: Oxygen isotopic evidence for the depth stratification of Tertiary and Cretaceous planktic foraminifera, Mar. Micropaleontol., 3, 175–196, 1978.
Dunkley Jones, T., Bown, P. R., and Pearson, P. N.: Exceptionally well preserved upper Eocene to lower Oligocene calcareous nannofossils (Prymnesiophyceae) from the Pande Formation (Kilwa Group), Tanzania, J. Syst. Palaeontol., 7, 359–411, https://doi.org/10.1017/S1477201909990010, 2009.
Edgar, K. M., Bohaty, S. M., Gibbs, S. J., Sexton, P. F., Norris, R. D., and Wilson, P. A.: Symbiont “bleaching” in planktic foraminifera during the Middle Eocene Climatic Optimum, Geology, 41, 15–18, https://doi.org/10.1130/G33388.1, 2013.
Edgar, K. M., Anagnostou, E., Pearson, P. N., and Foster, G. L.: Assessing the impact of diagenesis on δ11B, δ13C, δ18O, Sr/Ca and B/Ca values in fossil planktic foraminiferal calcite, Geochim Cosmochim. Ac., 166, 189–209, https://doi.org/10.1016/j.gca.2015.06.018, 2015.
Edgar, K. M., Hull, P. M., and Ezard, T. H.: Evolutionary history biases inferences of ecology and environment from δ13C but not δ18O values, Nat. Commun., 8, 1–9, https://doi.org/10.1038/s41467-017-01154-7, 2017.
Eggins, S., De Deckker, P., and Marshall, J.: Mg/Ca variation in planktonic foraminifera tests: implications for reconstructing palaeo-seawater temperature and habitat migration, Earth Plan. Sc. Lett., 212, 291–306, https://doi.org/10.1016/S0012-821X(03)00283-8, 2003.
Erez, J.: The source of ions for biomineralization in foraminifera and their implications for paleoceanographic proxies, Rev. Mineral. Geochem., 54, 115–149, https://doi.org/10.2113/0540115, 2003.
Ezard, T. H. G., Aze, T., Pearson, P. N., and Purvis, A.: Interplay between changing climate and species' ecology drives macroevolutionary dynamics, Science, 332, 349–351, https://doi.org/10.1126/science.1203060, 2011.
Fayolle, F. and Wade, B. S.: The evolution of Eocene planktonic foraminifera Dentoglobigerina, J. Syst. Palaeontol., 19, 333–376, https://doi.org/10.1080/14772019.2021.1904021, 2021.
Fehrenbacher, J. S., Russell, A. D., Davis, C. V., Gagnon, A. C., Spero, H. J., Cliff, J. B., Zhu, Z., and Martin, P.: Link between light-triggered Mg-banding and chamber formation in the planktic foraminifera Neogloboquadrina dutertrei, Nat. Commun., 8, 15441, https://doi.org/10.1038/ncomms15441, 2017.
Fraass, A., Kelly, D. C., and Peters, S. E.: Macroevolutionary history of the planktic foraminifera, Annu. Rev. Earth Pl. Sc., 43, 150112145716004, https://doi.org/10.1146/annurev-earth-060614-105059, 2014.
Frieling, J., Gebhardt, H., Huber, M., Adekeye, O. A., Akande, S. O., Reichart, G. J., Middelburg, J. J., Schouten, S., and Sluijs, A.: Extreme warmth and heat-stressed plankton in the tropics during the Paleocene-Eocene Thermal Maximum, Sci. Adv., 3, e1600891, https://doi.org/10.1126/sciadv.1600891, 2017.
Furbish, D. J. and Arnold, A. J.: Hydrodynamic strategies in the morphological evolution of spinose planktonic foraminifera, Oceanographic Literature Review, 3, 504–505, 1988.
Gaskell, D. E. and Hull, P. M.: Symbiont arrangement and metabolism can explain high δ13C in Eocene planktonic foraminifera, Geology, 47, 1156–1160, https://doi.org/10.1130/G46304.1, 2019.
Gaskell, D. E., Ohman, M. D., and Hull, P. M.: Zooglider-based measurements of planktonic foraminifera in the California Current System, J. Foramin. Res., 49, 390–404, 2019.
Grigoratou, M., Monteiro, F. M., Ridgwell, A., and Schmidt, D. N.: Investigating the benefits and costs of spines and diet on planktonic foraminifera distribution with a trait-based ecosystem model, Mar. Micropaleontol., 166, 102004, https://doi.org/10.1016/j.marmicro.2021.102004, 2021.
Hemleben, C.: Spine and pustule relationship in some Recent planktonic foraminifera, Micropaleontology, 21, 334–341, 1975.
Hemleben, C. and Olsson, R. K.: Wall textures of Eocene planktonic foraminifera, in: Atlas of Eocene Planktonic Foraminifera, edited by: Pearson, P. N., Olsson, R. K., Huber, B. T., Hemleben, C., and Berggren, W. A., Cushman Foundation for Foraminiferal Research, Special Publication 41, 47–68, 2006.
Hemleben, C., Brönniman, P., and Renz, H. H.: Ultramicroscopic shell and spine structure of some spinose planktonic Foraminifera, in: Proceedings of the First International Conference on Planktonic Microfossils, Geneva, 1967, edited by: Bronnimann, P. and Renz, H. H., E.J. Brill, Leiden, Vol. 2, 254–256, 1969.
Hemleben, C., Spindler, M., and Anderson, O. R.: Modern Planktonic Foraminifera, Springer, New York, 363 pp., 1989.
Hemleben, C., Muhlen, D., Olsson, R. K., and Berggren, W. A.: Surface texture and the first occurrence of spines in planktonic foraminifera from the early Tertiary, Geologisches Jahrbuch A, 128, 117–146, 1991.
Hemleben, C., Olsson, R. K., Premec Fucek, V., and Hernitz-Kucenjac, M.: Wall textures of Oligocene normal perforate planktonic Foraminifera, in: Atlas of Oligocene Planktonic Foraminifera, edited by: Wade, B. S., Olsson, R. K., Pearson, P. N., Huber, B. T., and Berggren, W. A., Cushman Foundation of Foraminiferal Research, Special Publication 46, 55–78, 2018.
Henehan, M. J., Edgar, K. M., Foster, G. L., Penman, D. E., Hull, P. M., Greenop, R., Anagnostou, E., and Pearson, P. N.: Revisiting the Middle Eocene Climatic Optimum “carbon cycle conundrum” with new estimates of atmospheric pCO2 from boron isotopes, Paleoceanogr. Paleocl., 35, e2019PA003713, https://doi.org/10.1029/2019PA003713, 2020.
Huber, B. T. and Leckie, R. M.: Planktic foraminiferal species turnover across deep-sea Aptian/Albian boundary sections, J. Foramin. Res., 41, 53–95, 2011.
Hull, P. M., Osborn, K. J., Norris, R. D., and Robison, B. H.: Seasonality and depth distribution of a mesopelagic foraminifer, Hastigerinella digitata, in Monterey Bay, California, Limnol. Oceanogr., 56, 562–576, https://doi.org/10.4319/lo.2011.56.2.0562, 2011.
Hull, P. M., Bohaty, S. M., Cameron, A., Coxall, H. K., D'haenens, S., De Vleeschouwer, D., Elder, L. E., Friedrich, O., Kerr, K., Turner, S. K., Kordesch, W. E. C., Morlua, K., Norris, P. D., Opdyke, B. N., Penman, D. E., Palike, H., Wilson, P. A., Sexton, P. F., Vaklenkamp, M., Wu, F., and Zachos, J. C.: Data report: coarse fraction record for the Eocene megasplice at IODP Sites U1406, U1408, U1409, and U1411, Proceedings of the Integrated Ocean Drilling Program, 342, 1–9, https://doi.org/10.2204/iodp.proc.342.203.2017, 2017.
John, E. H., Pearson, P. N., Coxall, H. K., Birch, H., Wade, B. S., and Foster, G. L.: Warm ocean processes and carbon cycling in the Eocene, Philos. T. R. Soc. A, 371, 20130099, https://doi.org/10.1098/rsta.2013.0099, 2013.
John, E. H., Wilson, J. D., Pearson, P. N., and Ridgwell, A.: Temperature-dependent remineralization and carbon cycling in the warm Eocene oceans, Palaeogeogr. Palaeocl., 413, 158–166, https://doi.org/10.1016/j.palaeo.2014.05.019, 2014.
Koutsoukos, E. A.: Phenotypic plasticity, speciation, and phylogeny in early Danian planktic foraminifera, J. Foramin. Res., 44, 109–142, 2014.
Liu, C. and Olsson, R. K.: On the origin of Danian normal perforate planktonic foraminifera from Hedbergella, J. Foramin. Res., 24, 61–74, 1994.
Loeblich Jr., A. R. and Tappan, H.: Suprageneric classification of the Rhizopodea, J. Paleontol., 35, 245–330, 1961.
Loeblich Jr., A. R. and Tappan, H.: Foraminiferal genera and their classification, New York, Van Nostrand Reinhold, 970 pp., 1988.
Luciani, V., Dickens, G. R., Backman, J., Fornaciari, E., Giusberti, L., Agnini, C., and D'Onofrio, R.: Major perturbations in the global carbon cycle and photosymbiont-bearing planktic foraminifera during the early Eocene, Clim. Past, 12, 981–1007, https://doi.org/10.5194/cp-12-981-2016, 2016.
Luciani, V., D'Onofrio, R., Dickens, G. R., and Wade, B. S.: Planktic foraminiferal response to early Eocene carbon cycle perturbations in the southeast Atlantic Ocean (ODP Site 1263), Global Planet. Change, 158, 119–133, https://doi.org/10.1016/j.gloplacha.2017.09.007, 2017.
McGowran, B.: Reclassification of early Tertiary Globorotalia, Micropaleontology, 14, 179–198, 1968.
Nicholas, C. J., Pearson, P. N., Bown, P. R., Jones, T. D., Huber, B. T., Karega, A., Lees, J. A., McMillan, I. K., O'Halloran, A., Singano, J. M., and Wade, B. S.: Stratigraphy and sedimentology of the Upper Cretaceous to Paleogene Kilwa Group, southern coastal Tanzania, J. Afr. Earth Sci., 45, 431–466, https://doi.org/10.1130/B26261.1, 2006.
Nicholas, C. J., Pearson, P. N., McMillan, I. K., Ditchfield, P. W., and Singano, J. M.: Structural evolution of southern coastal Tanzania since the Jurassic, J. Afr. Earth Sci., 48, 273–297, https://doi.org/10.1016/j.jafrearsci.2007.04.003, 2007.
Norris, R. D.: Symbiosis as an evolutionary innovation in the radiation of Paleocene planktonic foraminifera, Paleobiology, 22, 386–405, 1996.
Norris, R. D., Wilson, P. A., Blum, P. Fehr, A., Agnini, C., Bornemann, A., Boulila, S., Bown, P. R., Cournede, C., Friedrich, O., Ghosh, A. K., Hollis, C. J., Hull, P.M., Jo, K., Junium, C.K., Kaneko, M., Liebrand, D., Lippert, P. C., Liu, Z., Matsui, H., Moriya, K., Nishi, H., Opdyke, B. N., Penman, D., Romans, B., Scher, H. D., Sexton, P., Takagi, H., Kirtland Turner, S. Whiteside, J. H., Yamaguchi, T., and Yamamoto, Y.: Site U1408. Proceedings of the Integrated Ocean Drilling Program, 342, 91, https://doi.org/10.2204/iodp.proc.342.109.2014, 2014.
Olsson, R. K., Hemleben, C., Berggren, W. A., and Liu, C.: Wall texture classification of planktonic foraminifera genera in the lower Danian, J. Foramin. Res., 22, 195–213, 1992.
Olsson, R. K., Berggren, W. A., Hemleben, C., and Huber, B. T.: Atlas of Paleocene planktonic foraminifera, Smithsonian Contributions to Paleobiology, Vol. 85, 252 pp., 1999.
Olsson, R. K., Hemleben, C., Huber, B. T., and Bergrren, W. A.: Taxonomy, biostratigraphy, and phylogeny of Eocene Globigerina, Globoturborotalita, Subbotina, and Turborotalita, in: Atlas of Eocene Planktonic Foraminifera, edited by: Pearson, P. N., Olsson, R. K., Huber, B. T., Hemleben, C., and Berggren, W. A., Cushman Foundation, Special Publication 41, 111–168, 2006.
Parker, F. L.: Planktonic foraminiferal species in Pacific sediments, Micropaleontology, 8, 219–254, 1962.
Pearson, P. N.: Oxygen isotopes in foraminifera: overview and historical review, in: Reconstructing Earth's Deep-Time Climate – The State of the Art in 2012, edited by: Ivany, L. C. and Huber, B. T., Paleontological Society Papers, Vol. 18, 1–38, 2012.
Pearson, P. N. and Berggren, W. A.: Taxonomy, biostratigraphy, and phylogeny of Morozovelloides n. gen., in: Atlas of Eocene Planktonic Foraminifera, edited by: Pearson, P. N., Olsson, R. K., Huber, B. T., Hemleben, C., and Berggren, W. A., Cushman Foundation for Foraminiferal Research, Special Publication 41, 327–342, 2006.
Pearson, P. N. and Coxall, H. K.: Origin of the Eocene planktonic foraminifer Hantkenina by gradual evolution, Palaeontology, 57, 243–267, https://doi.org/10.1111/pala.12064, 2014.
Pearson, P. N. and Palmer, M. R.: Middle Eocene seawater pH and atmospheric carbon dioxide concentrations, Science, 284, 1824–1826, https://doi.org/10.1126/science.284.5421.1824, 1999.
Pearson, P. N. and Palmer, M. R.: Atmospheric carbon dioxide concentrations over the past 60 million years, Nature, 406, 695–699, https://doi.org/10.1038/35021000, 2000.
Pearson, P. N. and Wade, B. S.: Systematic taxonomy of exceptionally well-preserved planktonic foraminifera from the Eocene/Oligocene boundary of Tanzania, Cushman Foundation for Foraminiferal Research, Special Publication 45, 1–85, 2015.
Pearson, P. N., Shackleton, N. J., and Hall, M. A.: Stable isotope paleoecology of middle Eocene planktonic foraminifera and multi-species isotope stratigraphy, DSDP Site 523, South Atlantic, J. Foramin. Res., 23, 123–140, 1993.
Pearson, P. N., Ditchfield, P. W., Singano, J., Harcourt-Brown, K. G., Nicholas, C. J., Olsson, R. K., Shackleton, N. J., and Hall, M. A.: Warm tropical sea surface temperatures in the Late Cretaceous and Eocene epochs, Nature, 413, 481–487, https://doi.org/10.1038/35097000, 2001.
Pearson P. N., Nicholas C. J., Singano J. M., Bown P. R., Coxall H. K., van Dongen B. E., Huber B. T., Karega A., Lees J. A., Msaky, E., Pancost, R. D., Pearson, M., and Roberts, A. P.: Paleogene and Cretaceous sediment cores from the Kilwa and Lindi areas of coastal Tanzania: Tanzania Drilling Project Sites 1–5, J. Afr. Earth Sci., 39, 25–62, https://doi.org/10.1016/j.jafrearsci.2004.05.001, 2004.
Pearson, P. N., Olsson, R. K., Huber, B. T., Hemleben, C., and Berggren, W. A.: Atlas of Eocene planktonic foraminifera, Cushman Foundation for Foraminiferal Research, Special Publication 41, 513 pp., 2006.
Pearson, P. N., van Dongen, B. E., Nicholas, C. J., Pancost, R. D., Schouten, S., Singano, J. M., and Wade, B. S.: Stable warm climate through the Eocene epoch, Geology, 35, 211–214, https://doi.org/10.1130/G23175A.1, 2007.
Pearson, P. N., Evans, S. L., and Evans, J.: Effect of diagenetic recrystallization on the strength of planktonic foraminifer tests under compression, J. Micropalaeontol., 34, 59–64, https://doi.org/10.1144/jmpaleo2013-032, 2015.
Poole, C. R. and Wade, B. S.: Systematic taxonomy of the Trilobatus sacculifer plexus and descendant Globigerinoidesella fistulosa (planktonic foraminifera), J. Syst. Palaeontol., 17, 23, https://doi.org/10.1080/14772019.2019.1578831, 2019.
Premoli Silva, I., Wade, B. S., and Pearson, P. N.: Taxonomy, biostratigraphy, and phylogeny of Globigerinatheka and Orbulinoides, in: Atlas of Eocene Planktonic Foraminifera, edited by: Pearson, P. N., Olsson, R. K., Huber, B. T., Hemleben, C., and Berggren, W. A., Cushman Foundation for Foraminiferal Research, Special Publication 41, 169–211, 2006.
Reiss, Z.: The Bilamellidea, nov. superfam. and remarks on Cretaceous globorotaliids, Contrib. Cushman Found. Foram. Res., 8, 127–145, 1957.
Schiebel, R. and Hemleben, C.: Modern Planktic Foraminifera, Paläont. Z., 79, 135–148, https://doi.org/10.1007/BF03021758, 2005.
Schiebel, R. and Hemleben, C.: Planktic Foraminifers in the Modern Ocean, Berlin, Springer, 358 pp., ISBN 978-3-662-50297-6, 2017.
Sexton, P. F., Wilson, P. A., and Pearson, P. N.: Palaeoecology of late middle Eocene planktic foraminifera and evolutionary implications, Mar. Micropaleontol. 60, 1–16, https://doi.org/10.1016/j.marmicro.2006.02.006, 2006a.
Sexton, P. F., Wilson, P. A., and Pearson, P. N.: Microstructural and geochemical perspectives on planktic foraminiferal preservation: “Glassy” versus “Frosty”, Geochem. Geophy. Geosy., 7, Q12P19, https://doi.org/10.1029/2006GC001291, 2006b.
Shackleton, N. J. and Boersma, A.: The climate of the Eocene ocean, J. Geol. Soc. Lond., 138, 153–157, 1981.
Shaw, J. O., D'haenens, S., Thomas, E., Norris, R. D., Lyman, J. A., Bornemann, A., and Hull, P. M.: Photosymbiosis in planktonic foraminifera across the Paleocene-Eocene thermal maximum, Paleobiology, 47, 632–647, https://doi.org/10.1017/pab.2021.7, 2021.
Spero, H. J., Eggins, S. M., Russell, A. D., Vetter, L., Kilburn, M. R., and Hönisch, B.: Timing and mechanism for intratest Mg/Ca variability in a living planktic foraminifer, Earth Planet. Sc. Lett., 409, 32–42, https://doi.org/10.1016/j.epsl.2014.10.030, 2015.
Spezzaferri, S., Kucera, M., Pearson, P. N., Wade, B. S., Rappo, S., Poole, C. R., Morard, R., and Stalder, C.: Fossil and genetic evidence for the polyphyletic nature of the planktonic foraminifera “Globigerinoides”, and description of the new genus Trilobatus, PLoS One, 10, e0128108, https://doi.org/10.1371/journal.pone.0128108, 2015.
Spindler, M., Hemleben, C., Salomons, J. B., and Smit, L. P.: Feeding behavior of some planktonic foraminifers in laboratory cultures, J. Foramin. Res., 14, 237–249, 1984.
Subbotina, N. N.: Fossil foraminifera of the USSR, Globigerinidae, Hantkeninidae and Globorotaliidae, Trudy Vesooyznogo Neftyanogo Nauchno-Issledovatel'skogo Geologo-Razvedochnogo Instituta (VNIGRI), 296 pp. 1953.
Van Dongen, B. E., Talbot, H. M., Schouten, S., Pearson, P. N., and Pancost, R. D.: Well preserved Cretaceous and Paleogene biomarkers from the Kilwa area, Tanzania, Org. Geochem., 37, 539–557, https://doi.org/10.1016/j.orggeochem.2006.01.003, 2006.
Wade, B. S.: Planktonic foraminiferal biostratigraphy and mechanisms in the extinction of Morozovella in the late Middle Eocene, Mar. Micropaleontol., 51, 23–38, https://doi.org/10.1016/j.marmicro.2003.09.001, 2004.
Wade, B. S. and Hernitz Kucenjak, M.: Taxonomy, biostratigraphy, and phylogeny of Oligocene Acarinina, in: Atlas of Oligocene Planktonic Foraminifera, edited by: Wade, B. S., Olsson, R. K., Pearson, P. N., Huber, B. T., and Berggren, W. A., Cushman Foundation of Foraminiferal Research, Special Publication 46, 393–402, 2018.
Wade, B. S., Al-Sabouni, N., Hemleben, C., and Kroon, D.: Symbiont bleaching in fossil planktonic foraminifera, Evol. Ecol., 22, 253–265, https://doi.org/10.1007/s10682-007-9176-6, 2008.
Wade, B. S., Pearson, P. N., Berggren, W. A., and Pälike, H.: Review and revision of Cenozoic tropical planktonic foraminiferal biostratigraphy and calibration to the Geomagnetic Polarity and Astonomical Time Scale, Earth Sci. Rev., 104, 111–142, https://doi.org/10.1016/j.earscirev.2010.09.003, 2011.
Wade, B. S., Premec-Fucek, V., Kamikuri, S., Bartol, M., Luciani, V., and Pearson, P. N.: Successive extinctions of muricate planktonic foraminifera (Morozovelloides and Acarinina) mark the base Priabonian, Newsl. Stratigr., 45, 245–262, https://doi.org/10.1127/0078-0421/2012/0023, 2012.
Wade, B. S., Olsson, R. K., Pearson, P. N., Huber, B. T., and Berggren, W. A. (Eds.): Atlas of Oligocene Planktonic Foraminifera, Cushman Foundation, Special Publication 46, 528 pp., 2018a.
Wade, B. S., Pearson, P. N., Olsson, R. K., Fraass, A. J., Leckie, R. M., and Hemleben, C.: Taxonomy, biostratigraphy, and phylogeny of Oligocene and lower Miocene Dentoglobigerina and Globoquadrina, in: Atlas of Oligocene Planktonic Foraminifera, edited by: Wade, B. S., Olsson, R. K., Pearson, P. N., Huber, B. T., and Berggren, W. A., Cushman Foundation of Foraminiferal Research, Special Publication 46, 331–384, 2018b.
Wade, B. S., Aljahdali, M. H., Mufrreh, Y. A., Memesh, A. M., AlSoubhi, S. A., and Zalmout, I. S.: Upper Eocene planktonic foraminifera from northern Saudi Arabia: implications for stratigraphic ranges, J. Micropalaeontol., 40, 145–161, https://doi.org/10.5194/jm-40-145-2021, 2021.
Weinkauf, M. F. G., Siccha, M., and Weiner, A. K. M.: Reproduction dynamics of planktonic microbial eukaryotes in the open ocean, J. R. Soc. Interface, 19, 20210860, https://doi.org/10.1098/rsif.2021.0860, 2022.
Zachos, J. C., Stott, L. D., and Lohmann, K. C.: Evolution of early Cenozoic marine temperatures, Paleoceanography, 9, 353–387, 1994.





