the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Change in biodiversity and abundance of benthic foraminifera with distance from the Rainbow hydrothermal vent field, Mid-Atlantic Ridge
Hannah Krüger
Gerhard Schmiedl
Zvi Steiner
Zhouling Zhang
Eric P. Achterberg
Nicolaas Glock
In the vicinity of hydrothermal vent fields, unique habitats are observed that are influenced by hydrothermal fluids. Benthic foraminifera can be part of the communities found around these hydrothermal vent fields. They can form suitable indicators for different marine environmental conditions because their tests are often well preserved in the sediments. In this work, living (rose-bengal-stained) and dead benthic foraminifera from six sediment core tops were investigated with increasing distance from the Rainbow hydrothermal vent field at the Mid-Atlantic Ridge. The proximal-to-distal transect was sampled starting at ∼ 200 m distance from the active vents and followed the vent plume for ∼ 41 km. The biodiversity and abundance of benthic foraminifera tended to increase with distance from the Rainbow hydrothermal vent field, and there was a significant difference between the living and dead assemblage. The Shannon–Wiener diversity was lower at 0.2–1 km distance from the vent field with 2.3 (living) and 2.8 (dead) and showed higher constant values of 3.0 to 3.3 (living) and ∼ 3.6 (dead) from a distance of ∼ 2 km onwards. The population density of living benthic foraminifera showed a similar pattern to the biodiversity, while the density of empty foraminiferal tests was subject to strong fluctuations. Differences in the species composition between live and dead assemblages indicate environmental fluctuations, which could be triggered by seasonal nutrient pulses or brief contacts of the vent plume with the sediment. Species composition changed with distance from the black smokers. While miliolids dominated sediments closer to the vent field, hyaline perforate and agglutinated species constituted the major parts of the assemblages at greater distances. Thus, miliolids may be better adapted to the environment potentially influenced by hydrothermal vent fluids than the hyaline and agglutinated species. Specifically, miliolids seem to tolerate elevated porewater concentrations of copper, cobalt, and zinc and are possibly influenced by intrusions of acidic hydrothermal fluids. This result is in contrast to studies from other venting sites with acidic fluids, where agglutinated species dominate. High biodiversity and abundances of benthic foraminifera suggest that a diverse benthic ecosystem occurs below the distal Rainbow vent plume.
- Article
(6683 KB) - Full-text XML
-
Supplement
(586 KB) - BibTeX
- EndNote
Hydrothermal vent fields form unique habitats in the deep ocean (Gooday and Rathburn, 1999; Wirth, 2016; Bernhard et al., 2023). In such hydrothermal environments, chemoautotrophic microbes are often the main primary producers (Jannasch and Mottl, 1985; Zierenberg et al., 2000) that enable the growth of dense populations of organisms, such as tube worms and clams (Hashimoto et al., 2001; Gollner et al., 2007; Wirth, 2016; Bernhard et al., 2023), by living in symbiosis (Wirth, 2016; e.g. Okada et al., 2019). Some species are endemic to hydrothermal venting areas (Gollner et al., 2010; Okada et al., 2019), such as the foraminifer Abyssotherma pacifica (Brönnimann et al., 1989). Generally, deep-water benthic foraminifera are test-bearing heterotrophic protists that belong to the supergroup Rhizaria and inhabit large parts of the seafloor (Haq and Boersma, 1978). They can be part of hydrothermal communities (e.g. Molina-Cruz and Ayala-López, 1988; Van Dover et al., 1988; Brönnimann et al., 1989; Ayala-López and Molina-Cruz, 1994; Jonasson et al., 1995; Panieri, 2006). Benthic foraminifera are potentially useful indicators of marine environmental conditions, as their abundance and biodiversity are determined by food input, presence of oxygen, and hydro-sedimentary processes (Jorissen et al., 2007). They are distinguished by their test type. Agglutinated benthic foraminifera form their tests by cementing sediment particles, whereas calcifying foraminifera are divided into hyaline perforate (Class Globothalamea) and miliolid (Class Tubothalamea) forms. The difference is based on the different formation and arrangement of their calcium carbonate (CaCO3) crystals. Furthermore, some species of benthic foraminifera have an organic-walled test (Order Allogromiida). Gooday et al. (2013) described organic-walled foraminiferal species that inhabit empty tests of planktic foraminifera, based on sediment samples from the Mid-Atlantic Ridge, where they made an important contribution to the benthic foraminiferal assemblage.
Hydrothermal vents are associated with geothermal activity at submarine fissures where seawater is heated due to a steep thermal gradient and reacts with the surrounding rocks whereby metals and other elements are leached (Douville et al., 2002; German and Von Damm, 2003; Wirth, 2016). The elements in the emitted vent fluids may precipitate on the seafloor upon contact with the cold ocean bottom waters, in association with sulfides or iron oxy-hydroxides (Feely et al., 1991; Cave et al., 2002; Wirth, 2016). Hydrothermal fluids typically generate a plume that can extend for hundreds to thousands of kilometres (Resing et al., 2015; Wirth, 2016). Most of the sulfide minerals and parts of the metal oxides settle to the seafloor within the first few hundred metres from the vent. Smaller metal oxides and various elements that remain soluble upon mixing of the hydrothermal fluid and seawater are carried in the plume and may gradually sink over time. Figure 1 shows a schematic representation of a black smoker with the terms mentioned.
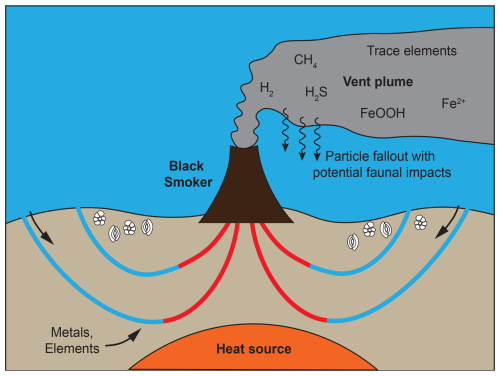
Figure 1A schematic representation of a black smoker showing seawater penetrating cracks in the mid-ocean ridge basalts. The seawater is thereafter heated by a steep geothermal gradient and thus reacts with the surrounding rocks, leaching metals and other elements. Upon venting back to the ocean, the hot hydrothermal fluids initially rise by 200–400 m above the vent depth due to their low density; at this point they cool and mix with enough seawater to obtain neutral buoyancy and continue to flow horizontally. Cooling of the hydrothermal fluid and interactions between the reduced hydrothermal fluid and oxidised seawater lead to precipitation of sulfide minerals and metal oxides.
A recent study investigating microbial communities in the water column at the Rainbow hydrothermal vent field showed that, when the vent plume was in direct contact with the sediment during vertical tidal migration, short-lived episodic community changes occurred (Haalboom et al., 2020). Haalboom et al. (2020) concluded that an increased export of microbial biomass from vent plumes may have an impact on other marine systems that are hospitable to chemolithoautotrophs.
In this study, the influences of hydrothermal activity on the biodiversity and abundance of benthic foraminifera were investigated by comparing the distribution of living and dead foraminiferal assemblages along a proximal-to-distal transect starting at a distance of ∼ 200 m to the Rainbow hydrothermal vent field. The transect followed the hydrothermal vent plume for ∼ 41 km. The foraminiferal assemblages were compared to nitrate, phosphate, and trace-metal concentrations in porewaters to investigate correlations between abundance, biodiversity, and porewater geochemistry. In addition, the work investigated whether there are foraminiferal indicator species for hydrothermal activity.
2.1 Study site
The Rainbow hydrothermal vent field is located in the North Atlantic Ocean at 36°13.80′ N and 33°54.14′ W (Fig. 2). It was discovered by German et al. (1996), and several studies have been conducted on the composition of the hydrothermal fluid and sediment related to the settling of particulate material (Cave et al., 2002; Edmonds and German, 2004; Haalboom et al., 2020). This vent field consists of 10 to 15 active black smokers located at ∼ 2300 m water depth (Seyfried et al., 2011). They are found on the western flank of a non-volcanic ridge at an offset between the South Alvin Mid-Atlantic Ridge (sAMAR) and Alvin Mid-Atlantic Ridge (AMAR) segments (Douville et al., 2002; Seyfried et al., 2011). The hydrothermal vent plume rises up to 300 m above the seafloor and tracks a canyon for 50 km, controlled by the local hydrodynamic regime and topography (Thurnherr and Richards, 2001; Thurnherr et al., 2002; Severmann et al., 2004 in Haalboom et al., 2020). The sampled proximal-to-distal transect begins at a distance of ∼ 200 m from the black smokers and follows the vent plume in a northeasterly direction for ∼ 41 km. The host rocks of the Rainbow hydrothermal vent field have an ultramafic composition with discharging hydrothermal fluids containing large amounts of hydrogen (H2) gas and iron (Fe), a moderate amount of methane (CH4), and a depleted hydrogen sulfide (H2S) content relative to other vent systems (Charlou et al., 2002; Seyfried et al., 2011; Zielinski et al., 2011; Haalboom et al., 2020). At the mouth of the hydrothermal vent chimneys, the fluids can reach temperatures of ∼ 365 °C and have a pH of ∼ 2.8 (Douville et al., 2002; Seyfried et al., 2011). The metals, including Fe and copper (Cu), precipitate from the fluid (Cave et al., 2002; Haalboom et al., 2020). The calcite saturation depth in the study area is slightly deeper than 4000 m, whereas the aragonite saturation depth lies at ∼ 2500 m (Chung et al., 2003). The sediment in the study area is carbonate-rich and contains mainly planktic foraminifera tests in the >125 µm fraction.
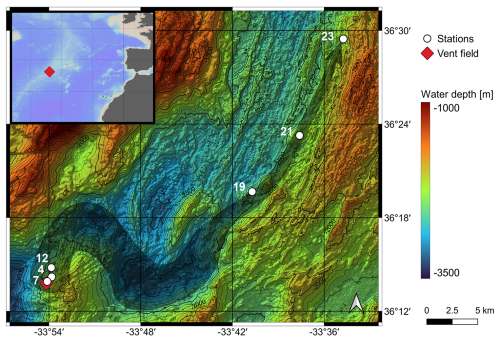
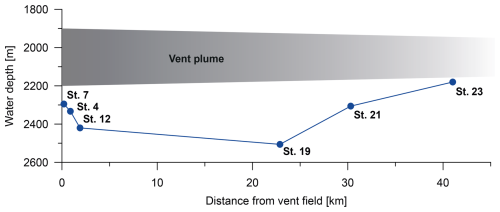
Figure 2Location of the Rainbow vent field (red diamond) and the sampled stations (white dots) with their assigned numbers. The grey arrow illustrates the vent plume. The map was created with sonar backscatter data from Eason et al. (2016) available at the Marine Geoscience Data System (Carbotte et al., 2004). The inset map shows the location of the Rainbow vent field in the North Atlantic Ocean. Ocean Data View was used to generate the inset map (Schlitzer, 2021).
2.2 Sampling
The samples were collected during the RV Meteor cruise M176/2 to the Rainbow non-buoyant hydrothermal plume as part of a GEOTRACES-compliant study (Fig. 2). Multiple sediment cores were collected along a transect using a multi-corer with a diameter of 10 cm (GEOMAR Helmholz Centre for Ocean Research Kiel). The multi-corer was equipped with a camera, and video observations were recorded. In this study, the uppermost 1 cm of one sediment core at each station was examined. The sampling transect included six stations with distances from the hydrothermal vent field ranging from 0.2–42 km and water depths between 2180 and 2508 m (Figs. 2 and 3). The vent plume was observed between 1900 and 2200 m depth. Sometimes the Rainbow vent plume may have had direct contact with the sediment due to vertical tidal migration (Haalboom et al., 2020).
2.3 Laboratory analyses
After sediment retrieval with the multi-corer, the samples were preserved in ethanol by adding a solution of rose bengal in ethanol (2 g L−1) to the sediment sample. The final concentration of ethanol in the preserved samples was ∼ 70 % (Schönfeld et al., 2012). Rose bengal stains organic material in a magenta colour by reacting with proteins in the cytoplasm (Walton, 1952). Thus, we infer that benthic foraminifera stained with rose bengal were living or recently dead at the time of sampling. Rose bengal staining is a common method for determining living foraminifera, but it has to be noted that, under anoxic conditions, the decay of protoplasm can proceed slowly and dead specimen may also be stained (Walker et al., 1974; Bernhard, 1988; Murray and Bowser, 2000; Bernhard, 2000; Schönfeld et al., 2012). The position of the sediment in the sample container was marked to later measure the volume. The samples were fractionated by wet sieving into 63–125 and > 125 µm fractions. The sample volume was determined by water volume measurement. The living benthic foraminifera were picked from the entire > 125 µm fraction of each station under a microscope. Afterwards, the > 125 µm fractions were split, and around 300 dead benthic foraminifera were picked from each sample. Total foraminiferal densities are likely underestimated by excluding the <125 µm fraction (Pawlowski et al., 1993; Gooday et al., 1998; Shepherd et al., 2007). However, the foraminiferal diversity should be reliably captured by the use of the >125 µm fraction. The greatest increase in species number and thus diversity usually occurs when the 125–250 µm fraction is included and when only a minor but sometimes considerable number of species (∼ 25 %) are confined to the 63–125 µm fraction (Gooday et al., 1998). The living and dead specimens were counted and identified following Jones (1994), Holbourn and Henderson (2002), Phleger et al. (1953), Schröder (1986), Hermelin and Scott (1985), and Gooday et al. (2013). The names of the species have been adapted according to the accepted taxonomical names from the World Register of Marine Species (WoRMS Editorial Board, 2022). Finally, images of abundant species were photographed with a Keyence VHX-6000 digital microscope and a Hitachi TM4000Plus scanning electron microscope (SEM) using backscattered electron (BSE) imaging.
The porewater of each sample was extracted in a cold laboratory kept at 4 °C, which is similar to the temperature of bottom water. Rhizon samplers were used for the porewater extraction (precleaned with 0.003 M hydrochloric acid and Milli-Q water; Steiner et al., 2018). The first 1 mL was discarded, and the following 5 mL was collected. The trace-metal samples were acidified by adding 20 µL of ultra-pure hydrochloride acid shortly after collection. The samples were later diluted at a ratio of 1 : 20 with 1 M double-distilled nitric acid before analyses. The measured nutrient concentrations in the surface water used for dilution of the porewater nutrient samples were reduced as blank from the measured porewater nutrient concentrations. Porewater nutrients were analysed during the cruise using a QUAATRO39 auto-analyser (Seal Analytics) following 1 : 6 dilution with nutrient-poor surface water. The nutrient analyses followed QUATRRO application method number Q-068-05 Rev. 11 for total oxidised nitrogen (TON) and Q-064-05 Rev. 8 for soluble reactive phosphate. Trace metals in the porewater and the water content and porosity of the sediment were measured at GEOMAR at a later time. Furthermore, trace elements were analysed in medium resolution (R=4000) for the masses 51V, 52Cr, 55Mn, 56Fe, 59Co, 60Ni, 63Cu, 95Mo, 98Mo, 137Ba, 138Ba, and 238U and in high resolution (R = 10 000) for 75As using high-resolution inductively coupled plasma mass spectrometry (HR-ICP-MS; Element-XR, Thermo Fisher). The HR-ICP-MS analyses were calibrated using standard additions of Inorganic Ventures single-element solutions to an acidified surface-water sample collected at the study site. Sediment samples destined for determination of porosity and the carbon content were sampled in pre-weighed vials and sent refrigerated to GEOMAR. The sediment was weighed before and after drying at 60 °C for 2–3 d for calculations of porewater content and porosity. The porosity and porewater content were calculated from the sediment mass loss upon drying, assuming solid sediment density of 2.70 g cm−2. Dried sediment was then powdered using agate mortar and pestle for analyses of its total carbon content using an EuroEA 3000 element analyser. Sediment organic carbon content was determined after acidification of the sediment using hydrochloric acid, and the inorganic carbon content was calculated as the difference between the total and organic carbon content.
2.4 Statistics
Statistical analyses were undertaken with the statistics software Past 4.03 (Hammer et al., 2001). The relationship between benthic foraminifera, environmental parameters, and community variations was analysed using multivariate statistics. Multivariate statistical methods were used to recognise relationships in the dataset by detecting important gradients. For multivariate analysis, canonical correspondence analysis (CCA) was executed (ter Braak, 1986). Prior to CCA, we tested whether there was a linear or unimodal distribution in the data using a detrended correspondence analysis (DCA). The most distant image along the first axis (gradient length) of the DCA was below 3 in both the living and the dead assemblages, indicating a linear distribution (Lepš and Šmilauer, 2003). In general, CCA samples are grouped according to their similarities or differences in a multidimensional space with axes that are described by eigenvalues (Murray, 2006). The variance of each axis results from these eigenvalues. The CCA combines the correspondence analysis with multiple linear regressions on the environmental variables and analyses them together with the dependent variables. Consequently, community variation can be directly related to environmental variations (ter Braak, 1986). In the interpretable coordinate system (CCA scatter plot), similar objects are close to each other, with species and sites indicated as dots and environmental variables indicated as vectors. For the CCA, the datasets of living and dead foraminiferal assemblages were reduced to a minimum mean relative abundance of 0.6 % for the six stations (, ), and congeners were lumped together. Environmental variables (water depth, porosity, nutrients, organic carbon content, carbonate content, and trace metals) were standardised to make them comparable. The standardisation was calculated as follows:
where z is the standardised score, is the mean, and SD is the standard deviation.
The biodiversity was described by the Shannon–Wiener index H(S), a calculation that reflects the biodiversity of an ecological assemblage including the species richness and abundance (Shannon and Weaver, 1963).
3.1 Distribution of benthic foraminifera species
A total of 90 different species of benthic foraminifera were identified in the living assemblage, and 126 different species were identified in the dead assemblage. Plate 1 shows the most abundant species of the total assemblage. In the following, the variable represents the mean of the six stations, with living and dead assemblages counted together (total assemblage). In the studied assemblages, agglutinated foraminifera were dominated by the genus Reophax ( = 3.8 %) and the species Rhizammina algaeformis ( = 3.0 %). Rhizammina algaeformis is a large branched foraminifer, usually only found in fragments due to subsampling, transport, and sieving of the sediment. In addition, Rhizammina spp., Rhabdammina spp., and Saccorhiza ramosa were also present in the samples. For comparison purposes, all fragments of the genera Rhizammina, Rhabdammina, and Saccorhiza were divided by 3 and then counted as whole individuals. This allows us to record the frequency of these species semi-quantitatively (Kurbjeweit et al., 2000; Heinz and Hemleben, 2003). Reophax was also present in the samples and represented by five different species. The most common species were R. bilocularis and R. scorpiurus.
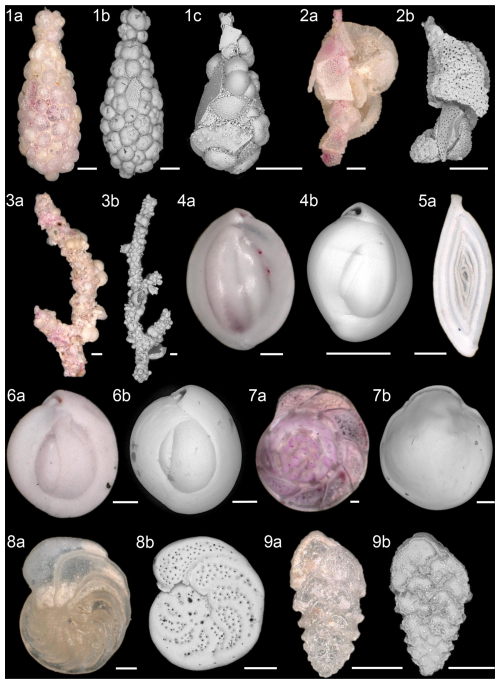
Plate 1Most abundant species of agglutinated (1–3), miliolid (4–6), and hyaline (7–9) benthic foraminifera. (1a–1c) Reophax bilocularis Flint, 1899; (2a–2b) Reophax scorpiurus Montfort, 1808; (3a–3b) Rhizammina algaeformis Brady, 1879; (4a–4b) Quinqueloculina weaveri Rau, 1948; (5) Spirosigmoilina pusilla (Earland, 1934); (6a–6b) Quinqueloculina seminulum (Linnaeus, 1758); (7a–7b) Hoeglundina elegans (d'Orbigny, 1826); (8a–8b) Cibicidoides wuellerstorfi (Schwager, 1866); and (9a–9b) Abditodentrix pseudothalmanni (Boltovskoy and Guissani de Kahn, 1981). Scale bar: 100 µm. Light microscopy (a) and an SEM (b, c) were used for the images.
Furthermore, the miliolids were dominated by Quinqueloculina weaveri ( = 7.9 %) and Spirosigmoilina pusilla ( = 7.5 %), with a relatively common appearance of Quinqueloculina seminulum ( %). The miliolid Triloculina trigonula only occurred at St. 4 and St. 7, and Sigmoilina sigmoidea occurred until a distance of ∼ 2 km (St. 12) from the vent field. Triloculina tricarinata was common in the dead assemblage, and its abundance increased with distance from the black smokers. Hoeglundina elegans ( = 7.1 %) was the most abundant hyaline species, followed by Cibicidoides wuellerstorfi ( %) and Abditodentrix pseudothalmanni ( %). The genera Cibicides and Cibicidoides were more common in the dead assemblages ( %) than in the living assemblages ( %). Besides C. wuellerstorfi, Cibicidoides mundulus occurred frequently, along with the hyaline genera Globocassidulina, Pullenia, and Gyroidina. The genera Fissurina and Parafissurina were common near the vent field. However, Laticarinina pauperata became more frequent with greater distance from the vent field. In addition, Hospitella fulva, an organic-walled foraminifer inhabiting planktic tests, was abundant in the living assemblage ( %), except in the sediment of St. 7. Hospitella fulva was the only identified organic-walled foraminifer due to a prominent tubular extension projecting from the host shell as described by Gooday et al. (2013). Scanning electron microscope images of H. fulva are shown in Plate 2. At St. 12, the composition of the benthic foraminifera assemblages differed from the other stations. Here, the living assemblage showed higher abundances of C. wuellerstorfi, Gavelinopsis translucens, Gyroidina polia, and Cornuloculina inconstans compared to the other stations. Living Osangularielloides rugosus and Hyperammina spp. only occurred at St. 12 and were frequent there. The dead assemblage at St. 12 was also different from the other stations, with high abundances of C. inconstans and S. ramosa but lower abundances of O. rugosus, G. translucens, and G. polia. A cluster of xenophyophores was observed at St. 12 and was sampled by the multi-corer. Figure 4 shows the xenophyophores directly before sampling, with a diameter of ∼ 2 cm and likely belonging to Syringammina sp. (Tendal and Gooday, 1981). Although xenophyophores are benthic foraminifera, they were not included in the diversity and abundance analyses. Due to their size difference, the observed xenophyophores were not comparable, and it would not have been possible to sample them accurately with the multi-corer. Besides benthic foraminifera, ostracods were abundant meiofauna in all samples. The samples contained mainly disarticulated ostracod valves but also a few rose-bengal-stained ostracods.
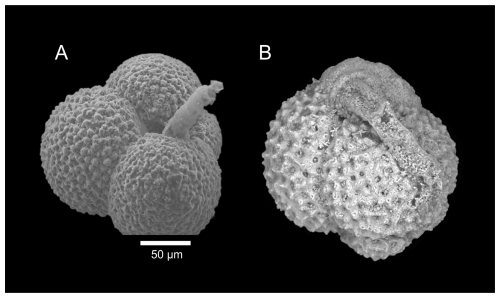
Plate 2SEM images of planktic foraminiferal tests inhabited by the organic-walled benthic foraminifer Hospitella fulva Rhumbler, 1911. A tubular extension of H. fulva projects from the aperture of the host test.
3.2 Distribution of main groups of benthic foraminifera
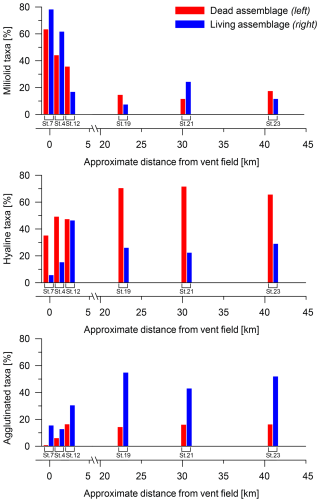
The main groups of benthic foraminifera showed remarkable differences along the transect and were divided by their test types in miliolids, agglutinated species, and hyalines. Figure 5 shows the distribution of the main groups with increasing distance from the vent field. Miliolids dominated the relative abundance near the vent field, while hyaline and agglutinated benthic foraminifera became relatively more abundant with increasing distance. The fauna of St. 7 consisted mainly of miliolids, with a relative abundance of 78.4 % in the living assemblage and of 63.6 % in the dead assemblage. Miliolids also dominated the living assemblage at St. 4 (miliolids 61.9 %), whereas hyaline foraminifera dominated the dead assemblage (hyaline 49.4 %, miliolids 44.3 %). At a ∼ 2 km distance from the vents, the relative abundance of miliolids decreased significantly. Hyaline living benthic foraminifera were rare near the vent field until a distance of ∼ 2 km (St. 12), where the highest relative abundance value along the transect was recorded (hyaline 46.6 %). The percentage of hyaline living specimens decreased to between 20 % and 30 % at St. 19, St. 21, and St. 23, whereas the hyaline dead specimens increased up to ∼ 70 %. Dead agglutinated foraminifera increased with distance from the vent field until ∼ 2 km. The locations between ∼ 23 km and ∼ 41 km are comparable to the ∼ 2 km location, with abundances of dead agglutinated specimens in a range between 14 % and 17 %. Living agglutinated specimens were more abundant than dead agglutinated specimens and dominated stations 19, 21, and 23 in a range of 30 % to 55 %.
3.3 Biodiversity and population density
Biodiversity and abundance of benthic foraminifera are represented by the Shannon–Wiener index, number of species, and population density, as illustrated in Fig. 6. The Shannon–Wiener diversity increased from 2.3 (living) and 2.8 (dead) at a distance of ∼ 200 m from the vent field to 3.3 (living) and 3.6 (dead) at ∼ 2 km distance from the vent field. At a greater distance, the diversity decreased slightly. It is noticeable that the Shannon–Wiener diversity in both the living and the dead assemblages initially exhibited a pronounced increase up to a distance of ∼ 2 km, after which it stabilised with a tendency towards a decline.
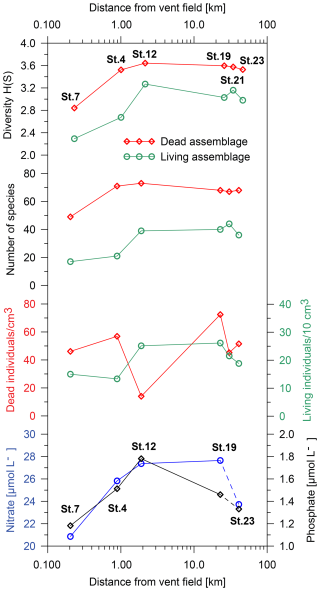
Figure 6Population density and density of empty foraminiferal tests (dead assemblage), Shannon–Wiener diversity index, number of species, and nitrate and phosphate concentrations with distance from the vent field. Note that the density of empty foraminiferal tests was calculated as individuals per cm3 and that the population density of living foraminifera was calculated as individuals per 10 cm3. Nitrate and phosphate were measured in the porewater extracted from the top sediment layers (St. 4, 1 cm; St. 7, 1 cm; St. 12, 2.5 cm; St. 19, 1 cm; St. 23, 0.7 cm). No measurements of nitrate and phosphate are available at St. 21.
The number of species in the living assemblage ranged from 17 different species at St. 7 and 21 species at St. 4 to about 40 species at the other stations. In the dead assemblage, 49 species were identified at St. 7, 71 species were identified at St. 4, and 73 species were identified at St. 12. Stations 4 and 12 were the most diverse stations along the transect in the dead assemblage. Stations 19, 21, and 23 showed slightly lower numbers of species, with 67 to 68 species in the dead assemblages.
The population density (i.e. standing stock) of living benthic foraminifera showed the lowest values at the two sites closest to the vent field. It increased from 15 individuals/10 cm3 at St. 7 and ∼ 13 individuals/10 cm3 at St. 4 to ∼ 25 individuals/10 cm3 at St. 12 and ∼ 26 individuals/10 cm3 at St. 19. It then decreased to ∼ 22 individuals/10 cm3 at St. 21 and ∼ 19 individuals/10 cm3 at St. 23. The density of empty foraminiferal tests initially increased from ∼ 46 individuals cm−3 at St. 7 to ∼ 57 individuals cm−3 at St. 4 but then decreased to 14 individuals cm−3 at St. 12. The highest value was observed at St. 19, with a density of ∼ 73 individuals cm−3. At St. 21 it decreased again to ∼ 45 individuals cm−3 before increasing to ∼ 52 individuals cm−3 at St. 23. In general, the population density of living benthic foraminifera was much lower than the density of empty foraminiferal tests (Fig. 6).
3.4 Trace metals
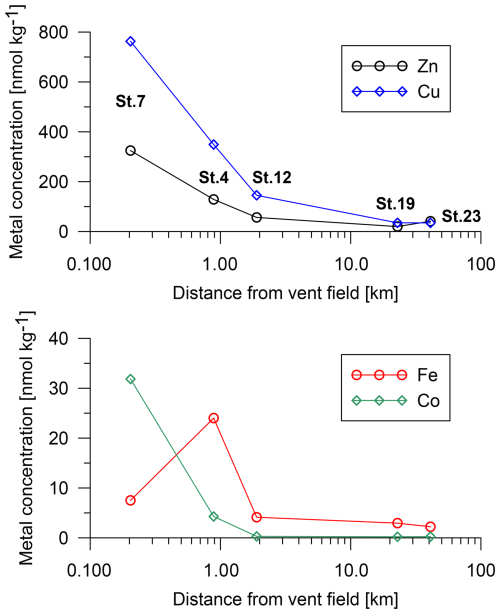
Porewater trace-metal concentrations were elevated near the vent field relative to the far-field stations (Fig. 7). At St. 7, the core-top porewater concentrations were 760 nmol kg−1 for Cu, 330 nmol kg−1 for Zn, and 32 nmol kg−1 for Co. At a greater distance (St. 19), Cu had a concentration of 35 nmol kg−1, Zn had a concentration of 20 nmol kg−1, and Co had a concentration of 0.22 nmol kg−1. Iron had a maximum concentration of 24 nmol kg−1 at 0.9 km distance.
3.5 Response to environmental variables
Porewater concentrations of nitrate (NO) and phosphate (PO) in the upper sediment increased significantly until ∼ 2 km distance from the vent field and decreased at further distance (Fig. 6). The sedimentary organic carbon content (Corg) was measured for St. 7 (0.39 %), St. 4 (0.24 %), St. 12 (0.35 %), and St. 23 (0.30 %), together with the calcium carbonate content at St. 7 (77 %), St. 4 (86 %), St. 12 (97 %), and St. 23 (92 %).
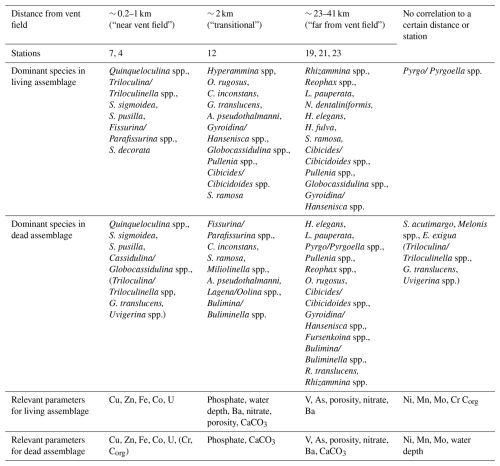
Scatter plots from canonical correspondence analysis (CCA) of the stained (Fig. 8) and dead (Fig. 9) assemblages indicate three different ecological communities along the transect. The CCA compares the distribution of benthic foraminifera at the different stations with trace metals, nitrate, phosphate, organic carbon content, carbonate content, water depth, and porosity. Species that belong to the different communities are listed in Table 1 together with stations where the communities are located and environmental parameters that dominated at these stations. A few species were omnipresent and could not be assigned to a specific community. Living Pyrgo/Pyrgoella spp. showed no direct connection with the three communities defined above. In the dead assemblage, S. acutimargo, Melonis spp., and E. exigua fluctuated discontinuously with distance from the Rainbow vent field, resulting in no obvious connection with the different communities. Dead Triloculina/ Triloculinella spp. were not clearly linked to the near-vent-field community but were clustered near G. translucens and Uvigerina spp., which are species that show no obvious trend along the transect. Dashed lines in the CCA scatter plots surround species that may also belong to the communities.
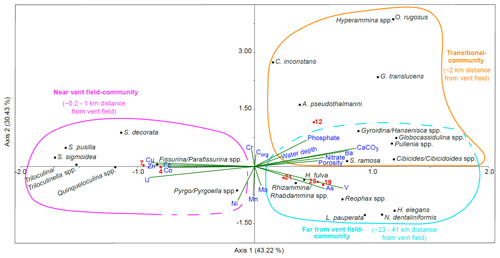
Figure 8Results of the canonical correspondence analysis (CCA) of living benthic foraminifera with reduced dataset and standardised environmental variables (water depth, porosity, nutrients, organic carbon, carbonate content, and porewater trace metals). Sampled stations are indicated with red dots and numbers. The CCA indicates the presence of three foraminiferal communities. The near-vent-field community represents St. 4 and St. 7 and contains miliolids, Fissurina/Parafissurina spp., and Spirillina decorata. Trace metals Cu, Zn, Co, U, Fe, and maybe Ni are associated with the near-vent-field community. The transitional community represents St. 12 with mainly hyalines such as Cibicides/Cibicidoides spp. and a connection to porosity, nitrate, Ba, water depth, and phosphate. Stations 19, 21, and 23 represent the far-from-vent-field community consisting of agglutinated and hyaline foraminifers and Hospitella fulva and connected to As and V.
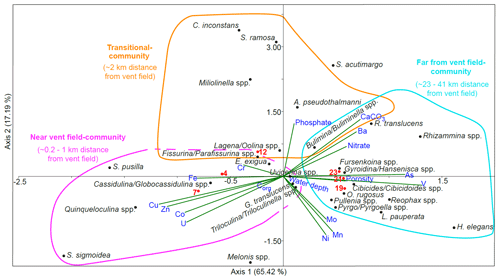
Figure 9Results of the canonical correspondence analysis (CCA) of dead benthic foraminifera with reduced dataset and standardised environmental variables (water depth, porosity, nutrients, organic carbon, carbonate content, and porewater trace metals). Sampled stations are indicated with red dots and numbers. Scatter plot with triplot amplitude of 1.5. The near-vent-field community of stations 4 and 7 is characterised by the dominance of miliolids and Cassidulina/Globocassidulina spp.; porewater at this environment is enriched with Cu, Zn, Co, U, Fe, and (Cr, Corg). The transitional community representing St. 12 includes Cornuloculina inconstans, Saccorhiza ramosa, Miliolinella spp., Abditodentrix pseudothalmanni, and others, and it is characterised by elevated porewater phosphate and sediment CaCO3 content. The far-from-vent-field community of stations 19, 21, and 23 includes hyalines, agglutinated foraminifera, and Pyrgo/Pyrgoella spp. which live in sediment with elevated porosity, As, V, nitrate, Ba, and CaCO3 compared to near-field cores. Melonis spp. and Spirophthalmidium acutimargo, along with porewater Ni, Mn, and Mo concentrations, show no obvious connection with the communities. The water depth shows no relevant relation with the species data.
Table 1Interpretation of the canonical correspondence analysis (CCA): correlating species, stations, and statistically relevant environmental parameters are listed by distance from vent field. The last column shows species and parameters with no obvious correlation with distance from vent field. Species and parameters in parentheses show a low correlation.
4.1 Factors influencing the composition of live and dead foraminiferal assemblages
The proximal-to-distal transect from the Rainbow hydrothermal vent field was divided into three regions. The first region located ∼ 0.2 to ∼ 1 km away from the Rainbow vent field contained St. 7 and St. 4 (“near vent field”); the second region located at a distance of ∼ 2 km from the vent field included St. 12 (“transitional”); and the third region located at a distance of ∼ 23 to ∼ 41 km from the vent field included St. 19, St. 21, and St. 23 (“far from vent field”). The transitional community is located at the only station where xenophyophores were observed. It is regarded as “transitional” because its communities differed from the other regions' and because it lies between the near-vent-field and far-from-vent-field communities. Its intermediate location suggests St. 12 likely sits outside the area that receives the main rain of sulfide minerals and metal oxides that follows the mixing of hydrothermal fluids and seawater but yet more plume fallout than the far-from-vent stations.
4.1.1 Taphonomic influences
Living and dead assemblages differed from each other. Agglutinated foraminifera were less abundant in the dead assemblage than in the living assemblage, probably due to low sample preservation quality. The preservation potential of foraminifera depends on the fragility of their tests and in general, agglutinated tests are more fragile than calcareous tests (Duros et al., 2012). Additionally, many genera such as Reophax and Rhizammina are agglutinated only with a degradable cement, often leading to their disappearance after death (Schröder, 1986; Schmiedl et al., 1997; Duros et al., 2012). This indicates that the hyaline foraminifera, which showed higher numbers in the dead than in the living assemblages, were likely overrepresented in the dead assemblage. The density of empty foraminiferal tests exhibited greater fluctuations than the population density in the living assemblage. The dead assemblage was likely subject to a greater degree of internal and external influences, such as bioturbation or hydro-sedimentary processes and possible physicochemical impacts (Martins et al., 2016). Significant differences can occur between the living and dead assemblages because the living assemblage only represents the moment of sampling, while the dead assemblage represents the accumulation of many generations over a longer time period (Murray, 1991, in Martins et al., 2016). Moreover, bioturbation and transport of tests often also cause such differences (Mackensen and Douglas, 1989; Murray, 2006; Martins et al., 2016). Finally, the shape of the canyon and slopes might have caused some relocation of tests in the study area.
4.1.2 Influence of the xenophyophore cluster
The density of empty foraminiferal tests was lowest at St. 12, which was located within a xenophyophore cluster. Xenophyophores are large, agglutinated foraminifers from the deep sea that can reach sizes of more than 20 cm (Gooday et al., 2011, 2020). Xenophyophores can be abundant megafaunal species in the deep sea, usually found under areas of high surface productivity or enhanced flux of organic matter (Levin and Thomas, 1988; Gooday et al., 2011). Similar clusters were observed by Tendal and Gooday (1981) and Gooday et al. (2011). Additionally, Ashford et al. (2014) carried out a habitat suitability modelling for xenophyophores and observed that an area of most suitable habitats for the xenophyophore Syringammina fragilissima includes the Mid-Atlantic Ridge close to the Azores, which is in close proximity to our sampling transect. The sediment material in the coarse fraction of St. 12 differed from the other stations because it contained xenophyophore remains. It also contained less planktic and dead meiobenthic foraminifera, explaining the low density of empty foraminiferal tests there (excluding the xenophyophores). The high abundance of living C. wuellerstorfi at St. 12 further implies different environmental conditions compared to the other stations where living Cibicides and Cibicidoides were rare. Cibicidoides wuellerstorfi prefers an elevated position above the sediment by attaching to sponge skeletons, stones, or other objects lying on the sediment surface (Lutze and Thiel, 1989; Burkett et al., 2015, 2016). Thus, C. wuellerstorfi can be underrepresented in living populations from samples of soft sediment (Lutze and Thiel, 1989). The xenophyophores at St. 12 could possibly serve as elevated substrate for C. wuellerstorfi. Other living species also showed their highest abundance at St. 12 (G. translucens, G. polia, and C. inconstans) or were even exclusively found at this station (O. rugosus and Hyperammina spp.), indicating the influence of different environmental conditions. Indeed, the measured environmental parameters indicate low porewater trace-metal concentrations at St. 12, comparable with the far-distance stations 19 and 23, a similar organic carbon content at all stations but higher nitrate concentrations at St. 12 and St. 19, and the highest phosphate along the transect at St. 12. Additionally, the highest carbonate content along the transect was measured at St. 12 (97 %).
The conditions in the transitional community might be favourable for benthic foraminifera. This is indicated by a relatively high abundance of living benthic foraminifera compared to most of the other stations and a high diversity in the living and dead assemblages. Only St. 19 showed a slightly higher abundance. Levin and Thomas (1988) found that the presence of xenophyophores can enhance the abundance of other organisms, such as isopods and nematodes. A similar positive effect may apply here at least to the occurrence of C. wuellerstorfi. Since the xenophyophores were exclusively found at St. 12, a patchy distribution of xenophyophores in the investigation area is consistent with our findings. Data from Gollner et al. (2007, 2010) suggest that foraminiferal abundances in vent habitats are patchy. This spatial heterogeneity (“patchiness”) is typical for deep-sea benthic communities and is caused by sediment characteristics, biogenic disturbances and structures, or the scattered distribution of food sources (Lejzerowicz et al., 2014).
4.1.3 Influence of environmental variability
Station 12 showed stronger differences in the species composition between the living and dead assemblages than the other sampling sites. This suggests that potential local environmental changes might have caused a change in the living fauna compared to the thanatocoenosis at this site. The xenophyophore cluster was probably relatively young compared to the underlying sediments and could have diluted the dead assemblage that accumulated there over hundreds of years. The growth rate of other xenophyophores is ∼ 1–2 cm/month (Gooday et al., 1993); thus, the xenophyophore cluster is probably not older than a few years, while the first 1 cm of sediment in the deep sea accumulates empty foraminifera tests from hundreds to thousands of years (Cave et al., 2002). Elevated epifaunal species such as C. wuellerstorfi respond to current flow and to the associated flux of suspended food particles (Gooday, 2003). This species seems to be adapted to high-current regimes (Wollenburg et al., 2021). Presumably, short-term current changes in the study area might cause differences in the food input and a response of benthic foraminifera leading to a change in the distribution of the foraminiferal assemblages. Additionally, the differences between abundance and species distribution at the different stations were larger in the living assemblage than in the dead assemblage. It is likely that there were short-term variations in the living assemblages that shifted the distribution of living organisms. Duros et al. (2012) suggested that differences between living and dead benthic fauna in the North Atlantic Ocean may reflect population dynamics and the temporal variability in the foraminiferal faunal composition. In bathyal regions, benthic foraminiferal assemblages can be influenced by seasonal changes in organic matter fluxes, whereas these are limited in the abyss (Gooday and Rathburn, 1999). However, seasonally varying organic matter fluxes also cause distribution changes in deep-sea foraminiferal faunas in some parts of the North Atlantic Ocean (Corliss et al., 2009). Species such as Epistominella exigua are related to fresh organic matter pulses, for example, coming from the spring bloom (Gooday, 1988; Schmiedl et al., 1997; Fontanier et al., 2003; Mojtahid et al., 2010). The species E. exigua was present in all samples of the dead assemblage of the current study area but only occurred in the living assemblage at St. 19. This suggests that the assemblages are linked to such organic matter pulses. It remains uncertain why living E. exigua were only found at this one station because a seasonal signal should have impacted all sites. Haalboom et al. (2020) showed that microbial communities at the Rainbow vent field were subject to short-lived episodic changes when the vent plume is in direct contact with the sediment. This might also have had an impact on the benthic foraminifera and could explain short-term community changes.
The Shannon–Wiener indices of benthic foraminifera, with values between 2.3 and 3.3 for the living assemblages and 2.8 and 3.6 for the dead assemblages, were moderate to high and increased with increasing distance from the vent field (e.g. compared to Buzas and Gibson, 1969; Schmiedl et al., 1997; Corliss et al., 2009). This was also observed in several studies which also indicated high diversity of benthic foraminifera assemblages in oxygenated deep-sea settings (e.g. Buzas and Gibson, 1969; Schmiedl et al., 1997; Gooday et al., 1998; Gooday, 1999). In deep-sea ecosystems, many environmental parameters are stable over time with no or few disturbances (“stability” hypothesis) (Buzas and Gibson, 1969). This can result in a high diversity even when oligotrophic conditions prevail (Buzas and Gibson, 1969; Schmiedl et al., 1997). The “stability” hypothesis could explain the high diversity at a greater distance from the vent field. While close to the vents, different disturbances due to the hydrothermal activity can likely result in a lower diversity. Food availability is typically a limiting factor for marine life in the deep sea, suggesting that population densities in deep-sea foraminiferal assemblages are generally low, because total foraminiferal standing stocks reflect food availability (Phleger, 1964, 1976; Douglas, 1981 in Gooday, 2003). Indeed, the biomass that might serve as a food source for benthic foraminifera potentially derives from organisms that thrive in the hydrothermal plume. For example, Nitrososphaeria (Archaea) and various proteobacteria make up large parts of the microbial community in the Rainbow vent plume (Haalboom et al., 2020). Our observed population densities are in a moderate range when compared to other deep-sea assemblages in the > 125 µm (or > 150 µm) fraction (e.g. Schmiedl et al., 1997; Wollenburg and Mackensen, 1998; Shepherd et al., 2007). Most hyaline and agglutinated species occurred in both the transitional and the far-from-vent-field communities. The differences in the species distribution between the transitional and the far-from-vent-field communities could have resulted from the presence of xenophyophores in the transitional community. The overlap in the community composition is probably because both were not strongly affected by disturbances due to the hydrothermal activity like the near vent stations.
4.2 Influence of hydrothermal activity on the benthic foraminifera
The biodiversity and population density of benthic foraminifera were low close to the Rainbow vent field. Different stress factors can cause an impoverished benthic foraminiferal fauna. For example, the hydrothermal fluids emit trace metals that can have an influence on the benthic foraminifera. Cu, Zn, and Co (partly Fe) in particular showed higher concentrations in the porewater in close proximity to the black smokers compared to the far-distance stations (Fig. 7). At high concentrations, these metals can be toxic to marine (micro-) organisms such as benthic foraminifera (Stankovic et al., 2014). In addition, the hydrothermal fluids are acidic (Douville et al., 2002), and a low pH in the proximity of the vents can potentially be a limiting factor for calcifying organisms like foraminifera (Brewer and Peltzer, 2009). Our closest station to the vents (St. 7) is at ∼ 200 m; such distance likely implies no indication of thermal anomalies compared to the open ocean. The tests of planktic foraminifera in the sediment at St. 7 and St. 4 exhibit poorer preservation in comparison to the other stations, suggesting that they were corroded by acidic porewaters. The surface sediment calcium carbonate content of the sediment dropped from 90 % in the far field stations to 77 % at St. 7. The decrease in %CaCO3 could be due to dilution of the CaCO3 with hydrothermal particles and dissolution of CaCO3. Nevertheless, the sedimentary sulfur content was low even at St. 7 (total sulfur content was <0.1 %). Hence, if we assume that the change in %CaCO3 was mostly due to dissolution with minor dilution, this represented a change in the ratio of other sediment constituents to CaCO3 from 1 : 9 to 1 : 3. In other words, the CaCO3 accumulation rate at St. 7 was only the accumulation rate at the far field stations. As the >125 µm fraction was mainly composed of planktic foraminifera whose rain rate should have been similar over this small region, this suggests that most of the CaCO3 dissolved at this site. However, given that the sediment at the closest station to the vent field was still dominated by CaCO3, the sediment must have reached equilibrium with calcite saturation, and the final pH should have been within the normal range for deep-sea environments.
It has been reported that agglutinating taxa are commonly dominant in other environments with low-pH or calcite-undersaturated waters, such as salt marshes (Edwards and Wright, 2015), deep-sea ecosystems below the calcite compensation depth (Mackensen et al., 1995), or other hydrothermal venting areas with acidic fluids (Jonasson et al., 1995; Jonasson and Schroeder-Adams, 1996; Panieri, 2006). In our study, hyaline and agglutinated benthic foraminifera were rare near the black smokers, suggesting a potentially negative influence of the hydrothermal activity. Instead, miliolids dominated near the vent site. It remains unclear why agglutinated foraminifera avoided the possibly acidic environments near the vent field. It has to be noted that our nearest sampling site was still at a distance of ∼ 200 m from the active vents and was not directly located at the vent site. In comparison to other vent fields, the hydrothermal fluids of the Rainbow vent field contained low concentrations of H2S and high concentrations of Cu (Douville et al., 2002). Since H2S and Cu can be toxic for marine organisms (Zierenberg et al., 2000; Stankovic et al., 2014), adaptations to their presence might explain differences between biodiversity and the abundance of benthic foraminifera at this and other venting sites. Miliolids might be better adapted to tolerate the extreme hydrothermal conditions than hyaline and agglutinated species. Ricketts et al. (2005, 2009) investigated the effects of CO2 hydrates on deep-sea foraminifera and showed that miliolids were more resistant to low pH. Thus, miliolids could be more resistant to acidic, metal-rich porewaters than hyaline and agglutinated species, which might explain why miliolids dominated near the vent field. Hyaline foraminifera precipitate low-Mg calcite, and sometimes aragonite, extracellularly after storage of calcium and carbonate in different intracellular pools (Todd and Blackmon, 1956; de Nooijer et al., 2009). Miliolids precipitate high-Mg calcite in the form of needles in the cell and form a framework with different orientation in the test wall, giving them a porcelaneous appearance (de Nooijer et al., 2009). Some miliolids form an outer organic lining when adding a new chamber, while hyaline and agglutinated foraminifera typically only have an inner organic lining (Berthold, 1976; Goldstein, 1999). The outer organic lining protects calcite crystals from dissolution from the surrounding medium (Berthold, 1976). Potentially, this mode of calcification allowed the examined miliolids in the near-vent-field community to precipitate their tests in a mildly corrosive environment.
Our data support the results of Molina-Cruz and Ayala-López (1988) and Ayala-López and Molina-Cruz (1994), who concluded that benthic foraminifera can inhabit environments close to hydrothermal vents. Similarly, Van Dover et al. (1988) reported benthic foraminifera as significant components of hydrothermal vent communities. However, the conditions close to the vent field seem to be stressful for the assemblages reflected by lower abundances and diversities but not harmful enough to cause disappearance. Some foraminiferal species with high relative abundances might even benefit. At a distance of ∼ 2 km from the vent field, the environmental conditions seem to be particularly favourable for benthic foraminifera, as certain species were found in high abundances and the highest biodiversity along the transect was recorded at this station. It is likely that the distance to the vents was far enough away from the hostile substances but influenced by food supply from the vents. Indeed, food availability should have been higher near the vent field due to the potential presence of chemoautotrophy. Lower porewater nitrate (20.9 µmol L−1) and phosphate (1.2 µmol L−1) concentrations near the vent field (∼ 200 m) were likely a consequence of reduced remineralisation rates of organic matter but were still rather high and indicated no nutrient limitation.
Benthic foraminiferal assemblages from the Rainbow hydrothermal vent field showed variations in diversity, population density, and species composition with increasing distance from the black smokers. The biodiversity and abundance of living benthic foraminifera were relatively low at ∼ 200 and ∼ 900 m distance from the black smokers but increased at a distance of ∼ 2 km. The locations between ∼ 23 and ∼ 41 km showed similar diversities, which were comparable to the ∼ 2 km location. The abundance peaked at ∼ 23 km distance and then slightly decreased again at the most distant stations from the vent field. The density of empty foraminiferal tests exhibited greater variability than the living population density with a relatively low value at ∼ 2 km distance from the vent field. The Shannon–Wiener diversity of the dead assemblage was slightly higher than that of the living assemblage and exhibited a similar proximal-to-distal trend.
Three benthic foraminiferal communities were identified along the investigated transect. The near-vent-field community between ∼ 200 m and ∼ 1 km from the black smokers was dominated by miliolids. At a greater distance from the vent field between ∼ 2 and ∼ 41 km, hyaline and agglutinated benthic foraminifera dominated the communities. The station at ∼ 2 km distance from the vent field differed in its species composition from the other stations and represented the highest biodiversity. The environmental conditions at this location may be particularly favourable for the growth of benthic foraminifera because the area still received material from the vents but had lower levels of potentially harmful substances. A xenophyophore cluster was present at this station and provided a particular habitat for the species found here. It remained unclear whether the location of this station or the presence of xenophyophores led to the differences compared to the other stations.
The hydrothermal activity probably influenced the near-vent-field community through different stressors. Miliolids seemed to demonstrate a greater tolerance to hydrothermal environmental conditions than hyaline and agglutinated benthic foraminifera. It can be hypothesised that the decline of hyaline and agglutinated foraminifera could be attributed to the acidic nature of the porewaters in the vicinity of black smokers. However, miliolids appeared to have greater resilience to such acidic conditions. Porewater pH measurements would have been necessary to prove this hypothesis. Besides the potential influence of acidic vent fluids, miliolid taxa seem to also tolerate other stress factors, such as the high trace-metal concentrations. Therefore, miliolids such as Quinqueloculina weaveri and Spirosigmoilina pusilla could possibly serve as indicator species for hydrothermal conditions in the study area. Hydrothermal vent fields can be “oases of life” in the mostly oligotrophic deep sea, where low abundances and a low input of organic matter predominate. The high biodiversity and abundance of benthic foraminifera near the Rainbow vent field suggest that a diverse ecological community is present there, with xenophyophores being a part of this community.
All data are available in the main text or in the Supplement.
The samples are stored at the Institute for Geology, University of Hamburg, Germany.
The supplement related to this article is available online at https://doi.org/10.5194/jm-44-193-2025-supplement.
NG and HK developed the concept. ZS, ZZ, EA, and NG did the sampling. HK carried out the sample preparation for micropalaeontological analyses. HK, GS, and NG did the taxonomic identification. ZS measured trace-metal concentrations; nitrate, phosphate, organic carbon, and carbonate content; and porosity. HK executed statistical analyses. HK wrote the main draft. All authors contributed to writing and editing the article.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We thank the captain and crew of RV Meteor for their help and support during cruise M176/2. We are highly grateful to Chris Galley and Lukas Klose for their efforts on the in situ mapping of the dispersing Rainbow hydrothermal plume. We thank Sarah Krüger for her support of multi-corer sampling. This work is a contribution to the Center for Earth System Research and Sustainability (CEN) of Universität Hamburg. Furthermore, we would like to thank Alexandra-Sophie Roy for English proofreading of the article. Finally, we thank the two anonymous reviewers and the editor Moriaki Yasuhara, whose comments improved the previous version of this article considerably.
This research has been supported by the Deutsche Forschungsgemeinschaft (grant nos. AC 217/4-1 and GL 999/3-1).
This paper was edited by Moriaki Yasuhara and reviewed by two anonymous referees.
Ashford, O. S., Davies, A. J., and Jones, D. O. B.: Deep-sea benthic megafaunal habitat suitability modelling: A global-scale maximum entropy model for xenophyophores, Deep-Sea Res. Pt. 1, 94, 31–44, https://doi.org/10.1016/j.dsr.2014.07.012, 2014.
Ayala-López, A. and Molina-Cruz, A.: Micropalaeontology of the hydrothermal region in the Guaymas Basin, Mexico, J. Micropalaeontol., 13, 133–146, https://doi.org/10.1144/jm.13.2.133, 1994.
Bernhard, J. M.: Postmortem vital staining in benthic foraminifera: duration and importance in population and distributional studies, J. Foramin. Res., 18, 143–146, 1988.
Bernhard, J. M.: Distinguishing live from dead foraminifera: methods review and proper applications, Micropaleontology, 46, 38–46, 2000.
Bernhard, J. M., Nomaki, H, Shiratori, T., Elmendorf, A., Yabuki, A., Kimoto, K., Tsuchiya, M., and Shimanaga, M.: Hydrothermal vent chimney-base sediments as unique habitat for meiobenthos and nanobenthos: Observations on millimeter-scale distributions, Front. Mar. Sci., 9, 1033381, https://doi/10.3389/fmars.2022.1033381, 2023.
Berthold, W.-U.: Biomineralisation bei milioliden Foraminiferen und die Matritzen-Hypothese, Naturwissenschaften, 63, 196–197, https://doi.org/10.1007/BF00624226, 1976.
Boltovskoy, E. and Giussani de Kahn, G.: Cinco nuevos taxones en Orden Foraminiferida. Comunicaciones des Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” e Instituto Nacional de Investigacion de las Ciencias Naturales, Hydrobiologia, 2, 43–51, 1981.
Brady, H. B.: Notes on some of the reticularian Rhizopoda of the “Challenger” Expedition; Part I. On new or little known arenaceous types, Q. J. Microsc. Sci., 19, 20–67, 1879.
Brewer, P. and Peltzer, E.: Limits to Marine Life, Science, 324, 347–348, https://doi.org/10.1126/science.1170756, 2009.
Brönnimann, P., Van Dover, C. L., and Whittaker, J. E.: Abyssotherma pacifica, n. gen., n. sp., a recent remaneicid (Foraminiferida, remaneicacea) from the East Pacific Rise, Micropaleontology, 35, 142–149, https://doi.org/10.2307/1485465, 1989
Burkett, A. M., Rathburn, A. E., Pérez, M. E., Levin, L. A., Cha, H., and Rouse, G. W.: Phylogenetic placement of Cibicidoides wuellerstorfi (Schwager, 1866) from methane seeps and non-seep habitats on the Pacific margin, Geobiology, 13, 44–52, https://doi.org/10.1111/gbi.12118, 2015.
Burkett, A. M., Rathburn, A. E., Pérez, M. E., Levin, L. A, and Martin, J. B.: Colonization of over a thousand Cibicidoides wuellerstorfi (foraminifera: Schwager, 1866) on artificial substrates in seep and adjacent off-seep locations in dysoxic, deep-sea environments, Deep-Sea Res. Pt. I, 117, 39–50, https://doi.org/10.1016/j.dsr.2016.08.011, 2016.
Buzas, M. A. and Gibson, T. G.: Species diversity: Benthonic foraminifera in western North Atlantic, Science, 163, 72–75, https://doi.org/10.1126/science.163.3862.72, 1969.
Carbotte, S. M., Arko, R., Chayes, D. N., Haxby, W., Lehnert, K., O'Hara, S., Ryan, W. B. F., Weissel, R. A., Shipley, T., Gahagan, L., Johnson, K., and Shank, T.: New Integrated Data Management System for Ridge2000 and MARGINS Research, EOS T. Am. Ggeophys. Un., 85, 553–560, https://doi.org/10.1029/2004EO510002, 2004.
Cave, R. R., German, C. R., Thomson, J., and Nesbitt, R.W.: Fluxes to sediments underlying the Rainbow hydrothermal plume at 36°14′ N on the Mid-Atlantic Ridge, Geochim. Cosmochim. Ac., 66, 1905–1923, https://doi.org/10.1016/S0016-7037(02)00823-2, 2002.
Charlou, J. L., Donval, J. P., Fouquet, Y., Jean-Baptiste, P., and Holm, N.: Geochemistry of high H2 and CH4 vent fluids issuing from ultramafic rocks at the Rainbow hydrothermal field (36°14′ N, MAR), Chem. Geol., 191, 345–359, https://doi.org/10.1016/S0009-2541(02)00134-1, 2002.
Chung, S.-N., Lee, K., Feely, R. A., Sabine, C. L., Millero, F. J., Wanninkhof, R., Bullister, J. L., Key, R. M., and Peng, T.-H.: Calcium carbonate budget in the Atlantic Ocean based on water column inorganic carbon chemistry, Global Biogeochem. Cy., 17, 1093, https://doi.org/10.1029/2002GB002001, 2003.
Corliss, B. H., Brown, C. W., Sun, X., and Showers, W. J.: Deep-sea benthic diversity linked to seasonality of pelagic productivity, Deep-Sea Res. Pt. I, 56, 835–841, https://doi.org/10.1016/j.dsr.2008.12.009, 2009.
de Nooijer, L. J., Toyofuku, T., and Kitazato, H.: Foraminifera promote calcification by elevating their intracellular pH, P. Natl. Acad. Sci., 106, 15374–15378, https://doi.org/10.1073/pnas.0904306106, 2009.
d'Orbigny, A. D.: Tableau méthodique de la classe des Céphalopodes, Annales des Sciences Naturelles, 7, 245–314, 1826.
Douglas, R. G.: Paleoecology of Continental Margin Basins: A modern case history from the borderland of Southern California, Depositional System of Active Continental Margin Basins-Short Course Notes, edited by: Douglas, R. G., Colburn, I. P., and Gorsline, D. S., 121–156, Pacific Section SEPM Short Course Notes, https://archives.datapages.com/data/pac_sepm/031/031001/pdfs/121.htm (last access: 26 July 2025), 1981.
Douville, E., Charlou, J. L., Oelkers, E. H., Bienvenu, P., Jove Colon, C. F., Donval, J. P., Fouquet, Y., Prieur, D., and Appriou, P.: The rainbow vent fluids (36°14′ N, MAR): the influence of ultramafic rocks and phase separation on trace metal content in Mid-Atlantic Ridge hydrothermal fluids, Chem. Geol., 184, 37–48, https://doi.org/10.1016/S0009-2541(01)00351-5, 2002.
Duros, P., Fontanier, C., de Stigter, H. C., Cesbron, F., Metzger, E., and Jorissen, F. J.: Live and dead benthic foraminiferal faunas from Whittard Canyon (NE Atlantic): Focus on taphonomic processes and paleo-environmental applications, Mar. Micropaleontol., 94–95, 25–44, https://doi.org/10.1016/j.marmicro.2012.05.004, 2012.
Earland, A.: Foraminifera, Part III, The Falklands sector of the Antarctic (excluding South Georgia), Discovery Reports, University Press, Cambridge, 208 pp., 1934.
Eason, D. E., Dunn, R. A., Canales, J. P., and Sohn, R. A.: Segment-scale variations in seafloor volcanic and tectonic processes from multibeam sonar imaging, Mid-Atlantic Ridge Rainbow region (35°45′–36°35′ N), Geochem. Geophy, Geosy., 17, 3560–3579, https://doi.org/10.1002/2016GC006433, 2016.
Edmonds, H. N. and German, C. R.: Particle geochemistry in the Rainbow hydrothermal plume, Mid-Atlantic Ridge, Geochim. Cosmochim. Ac., 68, 759–772, https://doi.org/10.1016/S0016-7037(03)00498-8, 2004.
Edwards, R. and Wright, A.: Chapter 13 – Foraminifera, in: Handbook of sea-level research, edited by: Shennan, I., Long, A. J., and Horton, B. P., John Wiley & Sons, Ltd., Chichester, 191–217, https://doi.org/10.1002/9781118452547, 2015.
Feely, R. A., Trefry, J. H., Massoth, G. J., and Metz, S.: A comparison of the scavenging of phosphorus and arsenic from seawater by hydrothermal iron oxyhydroxides in the Atlantic and Pacific Oceans, Deep-Sea Res., 38, 617–623, https://doi.org/10.1016/0198-0149(91)90001-V, 1991.
Flint, J. M.: Recent Foraminifera: a descriptive catalogue of specimens dredged by the U.S. Fish Commission steamer Albatross, Report of the United States National Museum, 249–349, 1899.
Fontanier, C., Jorissen, F. J., Chaillou, G., David, C., Anschutz, P., and Lafon, V.: Seasonal and interannual variability of benthic foraminiferal faunas at 550 m depth in the Bay of Biscay, Deep-Sea Res. Pt. I, 50, 457–494, https://doi.org/10.1016/S0967-0637(02)00167-X, 2003.
German, C. R. and Von Damm, K. L.: Hydrothermal Processes, Treatise on Geochemistry, 6, 181–222, https://doi.org/10.1016/B0-08-043751-6/06109-0, 2003.
German, C. R., Klinkhammer, G. P., and Rudnicki, M. D.: The Rainbow hydrothermal plume, 36°15′ N, MAR, Geophys. Res. Lett., 23, 2979–2982, https://doi.org/10.1029/96GL02883, 1996.
Goldstein, S. T.: Foraminifera: A biological overview, in: Modern Foraminifera, edited by: Sen Gupta, B., Kluwer Academic Publishers, 37–55, https://doi.org/10.1007/0-306-48104-9, 1999.
Gollner, S., Zekely, J., Govenar, B., Le Bris, N., Nemeschkal, H. L., Fisher, C. R., and Bright, M.: Tubeworm-associated permanent meiobenthic communities from two chemically different hydrothermal vent sites on the East Pacific Rise, Mar. Ecol.-Prog. Ser., 337, 39–49, https://doi.org/10.3354/meps337039, 2007.
Gollner, S., Riemer, B., Martínez Arbizu, P., Le Bris, N., and Bright, M.: Diversity of meiofauna from the 9°50′ N East Pacific Rise across a gradient of hydrothermal fluid emissions, PloS One, 5, E12321, https://doi.org/10.1371/journal.pone.0012321, 2010.
Gooday, A. J.: A response by benthic Foraminifera to the deposition of phytodetritus in the deep sea, Nature, 332, 70–73, https://doi.org/10.1038/332070a0, 1988.
Gooday, A. J.: Biodiversity of foraminifera and other protists in the deep sea: scales and patterns, Belg. J. Zool., 129, 61–80, 1999.
Gooday, A. J.: Benthic Foraminifera (Protista) as Tools in Deep-water Palaeoceanography: Environmental Influences on Faunal Characteristics, Adv. Mar. Biol., 46, 1–90, https://doi.org/10.1016/S0065-2881(03)46002-1, 2003.
Gooday, A. J. and Rathburn, A. E.: Temporal variability in living deep-sea benthic foraminifera: a review, Earth-Sci. Rev., 46, 187–212, https://doi.org/10.1016/S0012-8252(99)00010-0, 1999.
Gooday, A. J., Bett, B. J., and Pratt, D. N.: Direct observation of episodic growth in an abyssal xenophyophore (Protista), Deep-Sea Res. Pt. I, 40, 2131–2143, https://doi.org/10.1016/0967-0637(93)90094-J, 1993.
Gooday, A. J., Bett, B. J., Shires, R., and Lambshead, P. J. D.: Deep-sea benthic foraminiferal species diversity in the NE Atlantic and NW Arabian sea: a synthesis, Deep-Sea Res. Pt. II, 45, 165–201, https://doi.org/10.1016/S0967-0645(97)00041-6, 1998.
Gooday, A. J., Aranda da Silva, A., and Pawlowski, J.: Xenophyophores (Rhizaria, Foraminifera) from the Nazaré Canyon (Portuguese margin, NE Atlantic), Deep-Sea Res. Pt. II, 58, 2401–2419, https://doi.org/10.1016/j.dsr2.2011.04.005, 2011.
Gooday, A. J., Rothe, N, and Pearce, R. B.: New and poorly known benthic foraminifera (Protista, Rhizaria) inhabiting the shells of planktonic foraminifera on the bathyal Mid-Atlantic Ridge, Mar. Biol. Res., 9, 447–461, https://doi.org/10.1080/17451000.2012.750365, 2013.
Gooday, A. J., Durden, J. M., Holzmann, M., Pawlowski, J., and Smith, C. R.: Xenophyophores (Rhizaria, Foraminifera), including four new species and two new genera, from the western Clarion-Clipperton Zone (abyssal equatorial Pacific), Eur. J. Protistol., 75, 125715, https://doi.org/10.1016/j.ejop.2020.125715, 2020.
Haalboom, S., Price, D. M., Mienis, F., van Bleijswijk, J. D. L., de Stigter, H. C., Witte, H. J., Reichart, G.-J., and Duineveld, G. C. A.: Patterns of (trace) metals and microorganisms in the Rainbow hydrothermal vent plume at the Mid-Atlantic Ridge, Biogeosciences, 17, 2499–2519, https://doi.org/10.5194/bg-17-2499-2020, 2020.
Hammer, O., Harper, D. A. T., and Ryan, P. D.: PAST: Paleontological Statistics Software Package for Education and Data Analysis, Palaeontol. Electron., 4, 1–9, 2001.
Haq, B. U. and Boersma, A. (Eds.): Introduction to Mar. Micropaleontol., Elsevier, New York, 376 pp., ISBN 9780444826725, 1978.
Hashimoto, J., Ohta, S., Gamo, T., Chiba, H., Yamaguchi, T., Tsuchida, S., Okudaira, T., Watabe, H., Yamanaka, T., and Kitazawa, M.: First Hydrothermal Vent Communities from the Indian Ocean Discovered, Zool. Sci., 18, 717–721, https://doi.org/10.2108/zsj.18.717, 2001.
Heinz, P. and Hemleben, Ch.: Regional and seasonal variations of recent benthic deep-sea foraminifera in the Arabian Sea, Deep-Sea Res., 50, 435–447, https://doi.org/10.1016/S0967-0637(03)00014-1, 2003.
Hermelin, J. O. R. and Scott, D. B.: Recent Benthic Foraminifera from the Central North Atlantic, Micropaleontology, 31, 199–220, 1985.
Holbourn, A. E. and Henderson, A. S.: Re-illustration and Revised Taxonomy for Selected Deep-sea Benthic Foraminifers, Palaeontol. Electron., 4, 34 pp., https://palaeo-electronica.org/2001_2/foram/issue2_01.htm (last access: 26 July 2025), 2002.
Jannasch, H. W. and Mottl, M. J.: Geomicrobiology of Deep-Sea Hydrothermal Vents, Science, 229, 717–725, https://doi.org/10.1126/science.229.4715.717, 1985.
Jonasson, K. E. and Schroeder-Adams, C. J.: Encrusting agglutinated foraminifera on indurated sediment at a hydrothermal venting area on the Juan de Fuca Ridge, northeast Pacific Ocean, J. Foramin. Res., 26, 137–149, https://doi.org/10.2113/gsjfr.26.2.137, 1996.
Jonasson, K. E., Schröder-Adams, C. J., and Patterson, R. T.: Benthic foraminiferal distribution at Middle Valley, Juan de Fuca Ridge, a northeast Pacific hydrothermal venting site, Mar. Micropaleontol., 25, 151–167, 1995.
Jones, R. W. (Ed.): The Challenger Foraminifera, Oxford University Press, 149 pp., ISBN 9780198540960, 1994.
Jorissen, F. J., Fontanier, C., and Thomas, E.: Paleoceanographical proxies based on deep-sea benthic foraminiferal assemblage characteristics, Developments in Marine Geology, 1, 263–325, https://doi.org/10.1016/S1572-5480(07)01012-3, 2007.
Kurbjeweit, F., Schmiedl, G., Schiebel, R., Hemleben, C., Pfannkuche, O., Wallmann, K., and Schäfer, P.: Distribution, biomass and diversity of benthic foraminifera in relation to sediment geochemistry in the Arabian Sea, Deep-Sea Res. Pt. II, 47, 2913–2955, https://doi.org/10.1016/S0967-0645(00)00053-9, 2000.
Levin, L. A. and Thomas, C. L.: The ecology of xenophyophores (Protista) on eastern Pacific seamounts, Deep Sea Res., 35, 2003–2027, https://doi.org/10.1016/0198-0149(88)90122-7, 1988.
Lejzerowicz, F., Esling, P., and Pawlowski, J.: Patchiness of deep-sea benthic Foraminifera across the Southern Ocean: Insights from high-throughput DNA sequencing, Deep-Sea Res. Pt. II, 108, 17–26, https://doi.org/10.1016/j.dsr2.2014.07.018, 2014.
Lepš, J. and Šmilauer, P. (Eds.): Multivariate Analysis of Ecological Data using CANOCO, Cambridge: Cambridge University Press, 269 pp., https://doi.org/10.1017/CBO9781139627061, 2003.
Linnaeus, C. (Ed.): Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis, Editio decima, reformata, 1, 10th edn., Laurentius Salvius, Holmiae, 824 pp., https://doi.org/10.5962/bhl.title.542, 1758.
Lutze, G. F. and Thiel, H.: Epibenthic foraminifera from elevated microhabitats; Cibicidoides wuellerstorfi and Planulina ariminensis, J. Foramin. Res., 19, 153–158, https://doi.org/10.2113/gsjfr.19.2.153, 1989.
Mackensen, A. and Douglas, R. G.: Down-core distribution of live and dead deep-water benthic foraminifera in box cores from the Weddell Sea and the California continental borderland, Deep-Sea Res., 36, 879–900, https://doi.org/10.1016/0198-0149(89)90034-4, 1989.
Mackensen, A., Schmiedl, G., Harloff, J., and Giese, M.: Deep-Sea foraminifera in the South Atlantic Ocean: Ecology and assemblage generation, Micropaleontology, 41, 342–358, https://doi.org/10.2307/1485808, 1995.
Martins, M. V. A., Hohenegger, J., Frontalini, F., Miranda, P., Rodrigues, M. A. da C., and Dias, J. M. A.: Comparison between the dead and living benthic foraminiferal assemblages in Aveiro Lagoon (Portugal), Palaeogeogr. Palaeocl., 455, 16–32, https://doi.org/10.1016/j.palaeo.2016.05.003, 2016.
Mojtahid, M., Griveaud, C., Fontanier, C., Anschutz, P., and Jorissen, F. J.: Live benthic foraminiferal faunas along a bathymetrical transect (140–4800 m) in the Bay of Biscay (NE Atlantic), Les foraminifères benthiques vivants le long d'un transect bathymétrique (140–4800 m) dans le Golfe de Gascogne (Atlantique NE), Revue de micropaléontologie, 53, 139–162, https://doi.org/10.1016/j.revmic.2010.01.002, 2010.
Molina-Cruz, A. and Ayala-López, A.: Influence of the Hydrothermal Vents on the Distribution of Benthic Foraminifera from the Guayamas Basin, Mexico, Geo-Mar. Lett., 8, 49–56, https://doi.org/10.1007/BF02238006, 1988.
Montfort, P. (Ed.): Conchyliologie systématique et classification méthodique des coquilles, Paris, Schoell, Vol. 1, lxxxvii + 409 [1808] pp., Vol. 2, 676 + 16 [1810 (before 28 May)] pp., https://doi.org/10.5962/bhl.title.10571, 1808–1810.
Murray, J. W. (Ed.): Ecology and Palaeoecology of Benthic Foraminifera, Routledge, London, 408 pp., https://doi.org/10.4324/9781315846101, 1991.
Murray, J. W. (Ed.): Ecology and Applications of Benthic Foraminifera, Cambridge University Press, 426 pp., https://doi.org/10.1017/CBO9780511535529, 2006.
Murray, J. W. and Bowser, S. S.: Mortality, protoplasm decay rate, and reliability of staining techniques to recognize “living” foraminifera: a review, J. Foramin. Res., 30, 66–77, https://doi.org/10.2113/0300066, 2000.
Okada, S., Chen, C., Watsuji, T., Nishizawa, M., Suzuki, Y., Sano, Y., Bissessur, D., Deguchi. S., and Takai, K.: The making of natural iron sulfide nanoparticles in a hot vent snail, P. Natl. Acad. Sci., 116, 20376–20381, https:///doi.org/10.1073/pnas.1908533116, 2019.
Panieri, G.: The effect of shallow marine hydrothermal vent activity on benthic foraminifera (Aeolian Arc, Tyrrhenian Sea), J. Foramin. Res., 36, 3–14, https://doi.org/10.2113/36.1.3, 2006.
Pawlowski, J., Lee, J. J., and Gooday, A.: Microforaminifera – Perspective on a Neglected Group of Foraminifera, Arch. für Protistenkd., 143, 271–284, https://doi.org/10.1016/S0003-9365(11)80324-6, 1993.
Phleger, F. B.: Foraminiferal ecology and marine geology, Mar. Geol., 1, 16–43, https://doi.org/10.1016/0025-3227(64)90004-0, 1964.
Phleger, F. B.: Benthic foraminiferids as indicators of organic production in marginal marine areas, Maritime Sediments Special Publication, 1, 107–117, 1976.
Phleger, F. B., Parker, F. L., and Peirson, J. F.: North Atlantic Foraminifera, Reports of the Swedish Deep-Sea Expedition, 7, 122 pp., 1953.
Rau, W. W.: Foraminifera from the Porter Shale (Lincoln Formation), Grays Harbor County, Washington, J. Paleontol., 22, 152–174, https://www.jstor.org/stable/1299389 (last access: 26 July 2025), 1948.
Resing, J. A., Sedwick, P. N.; German, C. R., Jenkins, W. J., Moffett, J. W., Sohst, B. M., and Tagliabue, A.: Basin-scale transport of hydrothermal dissolved metals across the South Pacific Ocean, Nature, 523, 200–203, https://doi.org/10.1038/nature14577, 2015.
Rhumbler, L.: Die Foraminiferen (Talamophoren) der Plankton-Expedition, Zugleich Entwurf eines natürlichen Systems der Foraminiferen auf Grund selektionistischer und mechanisch-physiologischer Faktoren, Ergebnisse der Plankton-Expedition der Humboldt-Stiftung, 3, 331 pp., https://www.biodiversitylibrary.org/page/2132592 (last access: 27 July 2025), 1911.
Ricketts, E. R., Kennett, J. P., Hill, T. M., and Barry, J. P.: Effects of CO2 hydrate on deep-sea foraminiferal assemblages, Proceedings of the Fifth International Conference on Gas Hydrates, Trondheim, Norway, 3, 839–847, 2005.
Ricketts, E. R., Kennett, J. P., Hill, T. M., and Barry, J. P.: Effects of carbon dioxide sequestration on California margin deep-sea foraminiferal assemblages, Mar. Micropaleontol., 72, 165–175, https://doi.org/10.1016/j.marmicro.2009.04.005, 2009.
Schlitzer, R.: Ocean Data View, https://odv.awi.de (last access: 14 December 2022), 2021.
Schmiedl, G., Mackensen, A., and Müller, P. J.: Recent benthic foraminifera from the eastern South Atlantic Ocean: Dependence on food supply and water masses, Mar. Micropaleontol., 32, 249–287, 1997.
Schönfeld, J., Alve, E., Geslin, E., Jorissen, F., Korsun, S., and Spezzaferri, S.: The FOBIMO (FOraminiferal BIo-MOnitoring) initiative-Towards a standardised protocol for soft-bottom benthic foraminiferal monitoring studies, Mar. Micropaleontol., 94, 1–13, https://doi.org/10.1016/j.marmicro.2012.06.001, 2012.
Schröder, C. J. (Ed.): Deep-water arenaceous foraminifera in the northwest Atlantic Ocean, Canadian Technical Report of Hydrography and Ocean Science, 71, 191 pp., Fisheries and Oceans, Canada, http://hdl.handle.net/10222/75109 (last access: 26 July 2025), 1986.
Schwager, C.: Fossile Foraminiferen von Kar Nikobar. Reise der Österreichischen Fregatte Novara um die Erde in den Jahren 1857, 1858, 1859 unter den Befehlen des Commodore B. von Wüllerstorf-Urbair, Geologischer Theil (Zweite Abtheilung, Paläontologische Mittheilungen), 2, 187–268, 1866.
Severmann, S., Johnson, C. M., Beard, B. L., German, C. R., Edmonds, H. N., Chiba, H., and Green, D. R. H.: The effect of plume processes on the Fe isotope composition of hydrothermally derived Fe in the deep ocean as inferred from the Rainbow vent site, Mid-Atlantic Ridge, 36°14′ N, Earth Planet. Sc. Lett., 225, 63–76, https://doi.org/10.1016/j.epsl.2004.06.001, 2004.
Seyfried Jr., W. E., Pester, N. J., Ding, K., and Rough, M.: Vent fluid chemistry of the Rainbow hydrothermal system (36° N, MAR): Phase equilibria and in situ pH controls on subseafloor alteration processes, Geochim. Cosmochim. Ac., 75, 1574–1593, https://doi.org/10.1016/j.gca.2011.01.001, 2011.
Shannon, C. E. and Weaver, W. W. (Eds.): The mathematical theory of communications, University of Illinois Press, Urbana, 144 pp., ISBN 9780252725487, 1963.
Shepherd, A. S., Rathburn, A. E., and Pérez, M. E.: Living foraminiferal assemblages from the Southern California margin: A comparison of the >150, 63–150, and >63 µm fractions, Mar. Micropaleontol., 65, 54–77, https://doi.org/10.1016/j.marmicro.2007.06.001, 2007.
Stankovic, S., Kalaba, P., and Stankovic, A. R.: Biota as toxic metal indicators, Environ. Chem. Lett., 12, 63–84, https://doi.org/10.1007/s10311-013-0430-6, 2014.
Steiner, Z., Lazar, B., Erez, J., and Turchyn, A. V.: Comparing Rhizon samplers and centrifugation for pore-water separation in studies of the marine carbonate system in sediments, Limnol. Oceanogr.-Meth., 16, 828–839, https://doi.org/10.1002/lom3.10286, 2018.
Tendal, O. S. and Gooday, A. J.: Xenophyophoria (Rhizopoda, Protozoa) in bottom photographs from the bathyal and abyssal NE Atlantic, Oceanol. Acta, 4, 415–422, 1981.
ter Braak, C. J. F.: Canonical Correspondence Analysis: A New Eigenvector Technique for Multivariate Direct Gradient Analysis, Ecology, 67, 1167–1179, https://doi.org/10.2307/1938672, 1986.
Thurnherr, A. M. and Richards, K. J.: Hydrography and high-temperature heat flux of the Rainbow hydrothermal site (36°14′ N, Mid-Atlantic Ridge), J. Geophys. Res., 106, 9411–9426, https://doi.org/10.1029/2000JC900164, 2001.
Thurnherr, A. M., Richards, K. J., German, C. R., Lane-Serff, G. F., and Speer, K. G.: Flow and mixing in the rift valley of the Mid-Atlantic Ridge, J. Phys. Oceanogr., 32, 1763–1778, https://doi.org/10.1175/1520-0485(2002)032<1763:FAMITR>2.0.CO;2, 2002.
Todd, R. and Blackmon, P.: Calcite and Aragonite in Foraminifera, J. Paleontol., 30, 217–219, 1956.
Van Dover, C. L., Berg Jr., C. J., and Turner, R. D.: Recruitment of marine invertebrates to hard substrates at deep-sea hydrothermal vents on the East Pacific Rise and Galapagos spreading center, Deep-Sea Res., 35, 1833–1849, https://doi.org/10.1016/0198-0149(88)90052-0, 1988.
Walker, D. A., Linton, A. E., and Schafer, C. T.: Sudan Black B: A superior stain to Rose Bengal for distinguishing living from nonliving foraminifera, J. Foramin. Res., 4, 205–215, 1974.
Walton, W.: Techniques for recognition of living foraminifera, Contribution of Cushman, Foundation Foraminiferal Research, 3, 56–60, 1952.
Wirth, R.: Colonization of Black Smokers by Hyperthermophilic Microorganisms, Trends Microbiol., 25, 92–99, https://doi.org/10.1016/j.tim.2016.11.002, 2016.
Wollenburg, J. E. and Mackensen, A.: Living benthic foraminifers from the central Arctic Ocean: faunal composition, standing stock and diversity, Mar. Micropaleontol., 34, 153–185, https://doi.org/10.1016/S0377-8398(98)00007-3, 1998.
Wollenburg, J. E., Bijma, J., Cremer, C., Bickmeyer, U., and Zittier, Z. M. C.: Permanent ectoplasmic structures in deep-sea Cibicides and Cibicidoides taxa – long-term observations at in situ pressure, Biogeosciences, 18, 3903–3915, https://doi.org/10.5194/bg-18-3903-2021, 2021.
WoRMS Editorial Board: World Register of Marine Species, VLIZ, https://www.marinespecies.org (last access: 30 November 2022), https://doi.org/10.14284/170, 2022.
Zielinski, F. U., Gennerich, H.-H., Borowski, C., Wenzhöfer, F., and Dubilier, N.: In situ measurements of hydrogen sulfide, oxygen, and temperature in diffuse fluids of an ultramafic-hosted hydrothermal vent field (Logatchev, 14°45′ N, Mid-Atlantic Ridge): Implications for chemosymbiotic bathymodiolin mussels, Geochem. Geophy. Geosy., 12, Q0AE04, https://doi.org/10.1029/2011GC003632, 2011.
Zierenberg, R. A., Adams, M. W. W., and Arp, A. J.: Life in extreme environments: Hydrothermal vents, P. Natl. Acad. Sci., 97, 12961–12962, https://doi.org/10.1073/pnas.210395997, 2000.