the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Modified cleaning method for biomineralized components
Hideto Tsutsui
Richard W. Jordan
The extraction and concentration of biomineralized components from sediment or living materials is time consuming and laborious and often involves steps that remove either the calcareous or siliceous part, in addition to organic matter. However, a relatively quick and easy method using a commercial cleaning fluid for kitchen drains, sometimes combined with a kerosene soaking step, can produce remarkable results. In this study, the method is applied to sediments and living materials bearing calcareous (e.g., coccoliths, foraminiferal tests, holothurian ossicles, ichthyoliths, and fish otoliths) and siliceous (e.g., diatom valves, silicoflagellate skeletons, and sponge spicules) components. The method preserves both components in the same sample, without etching or partial dissolution, but is not applicable to unmineralized components such as dinoflagellate thecae, tintinnid loricae, pollen, or plant fragments.
- Article
(3828 KB) - Full-text XML
-
Supplement
(8008 KB) - BibTeX
- EndNote
Extracting and cleaning microfossils from sediments or mineralized components from living materials is often labor intensive and time consuming, and microscopic observation at each step is sometimes impractical, or in the case of dangerous chemicals, not advisable. Science often requires speed or efficient recovery, but usually it is difficult to combine both in a single method. Hence, a variety of methods have been developed to satisfy one or the other requirement. These include the use of sodium sulfate, sodium hydroxide, boron, hydrochloric acid, zinc chloride, hydrogen fluoride, hydrogen peroxide, acetone, ethanol, liquid nitrogen, potassium permanganate, hot water, and naphtha (e.g., Franke, 1922; Bourdon, 1962; Nishida, 1969; Hanken, 1979; Faegri et al., 1989; Shiono and Jordan, 1995; Lirer, 2000; Nielsen and Jakobsen, 2004; Abrantes et al., 2005; Remin et al., 2012; Jarochowska et al., 2013; Ikeya and Chinzei, 2007; Oda and Sato, 2013). Some extraction methods may require particular pieces of equipment, e.g., a sand bath, water bath, ultrasonic bath, dry oven, refrigerator, stirrer, settling chamber, or centrifuge. In addition, some chemicals require registration and complicated safety protocols and may be expensive to purchase and/or discard. Many methods require days or a week to complete the processing, whereby the sample solution needs time to settle in between decanting and washing steps. For instance, the potassium permanganate and hydrochloric acid method used by Shiono and Jordan (1995) for diatom preparation may take days to complete, while the nonacidic treatment method of Jarochowska et al. (2013) requires over 10 days. Potassium permanganate is known as a regulated pollutant, anti-infective medicine for dermatological problems (World Health Organization, 2015), and as a category 1 oxidizing substance (Fire Service Act in Japan, 2008). If possible, chemical processing should avoid these hazardous chemicals. Of course, the hydrochloric acid step in Shiono and Jordan (1995) or hydrogen peroxide cleaning step by Abrantes et al. (2005) dissolves the calcium carbonate component, e.g., coccoliths, holothurian ossicles, pteropod shells, and foraminiferal tests. Conversely, palynologists often use hydrogen fluoride when they wish to get rid of biogenic silica (e.g., radiolarians, diatoms, sponge spicules) in their samples, although hydrogen fluoride is known to dissolve both crystalline and amorphous silica (Liang and Readey, 1987).
Nishida (1969) has already documented a separation and enrichment method for coccoliths and silicoflagellates in the Houjyuji diatomites of Noto Peninsula, Japan, using a centrifuge and showed that no damage occurred to the calcareous nannofossils using this sodium hypochlorite extraction method.
Nagumo (1995) reported a preparation method for diatoms using a kitchen drain cleaning fluid (Pipe Unish produced by SC Johnson). This method dissolves the conjugated proteins of diatoms and can reduce and easily control the cleaning process time without any special chemicals (Nagumo, 1995). Similarly, Yoshida et al. (2006) extracted holothurian ossicles using a household bleaching agent (Haiter produced by Kao), which contains sodium hydroxide, sodium hypochlorite, and a polyoxyethylene alkyl ether sulfate sodium salt. Sodium hydroxide dissolves the proteins in the holothurian skin.
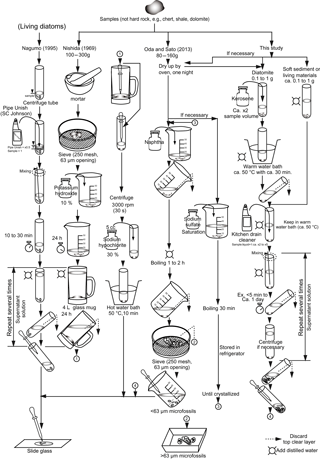
Figure 1Illustration of the extraction methods by Nagumo (1995), Nishida (1969), Oda and Sato (2013), and this study.
Oda and Sato (2013) summarized a useful extraction method for siliceous (e.g., radiolarians, diatoms) and calcareous (e.g., coccoliths, foraminifers) microfossils in sediments using sodium sulfate with naphtha. The advantage of this method is the rapid separation of the microfossils from the sediments. However, naphtha is difficult to purchase because it is registered as a category 4, class 1 oil in hazardous materials under the Fire Service Act in Japan (2008). Also, this method has some possibility of damaging the coccoliths during the cooling tension of sodium sulfate.
In summary, these three methods (Nishida, 1969; Nagumo, 1995; Yoshida et al., 2006; see Fig. 1), as well as the one using acetic acid (Jarochowska et al., 2013), are useful for the preliminary extraction of the sample from the sediment.
Therefore, in this study, the methods of Nishida (1969) and Nagumo (1995) were modified and improved in order to (1) provide a faster, easier, and simpler process; (2) reduce the quantity of chemicals needed for extraction and avoid the use of hazardous chemicals; and (3) reduce the damage to calcareous microfossils (e.g., coccoliths) during the chemical extraction.
2.1 Materials
In this study, a wide range of materials were collected from various places, including diatom-bearing sediments (Supplement Fig. S1a–b), living holothurians (Fig. S1c, e), fish otoliths (Fig. S1d), and marine lake sediments (Fig. S1e). A diatomite sample of early Eocene age from Mors, Denmark (e.g., Chambers et al., 2003; Collins et al., 2005; Jørgensen et al., 2005), and a diatom-bearing sample of late Eocene–Oligocene age from Oamaru, New Zealand (e.g., Lee et al., 1997; Novitski and Kociolek, 2005), were obtained from outcrops. Of the living holothurians from nearshore environments, one was collected from Peyrefite Bay in France, others were from Yaese-cho, Shimajiri, and Taketomi Island in Okinawa; Guam; or Bohol in the Philippines. The samples from the French coast and from Bohol were dried in natural sunlight, while those from Okinawa and Guam were soaked in 95 % ethyl alcohol. The sailfin sandfish (Arctoscopus japonicus) were caught in two places: off Shonai, Yamagata, and Karo, Tottori, Japan. The marine lake sediments from Jellyfish Lake (JFL) on Mecherchar Island (also known as Eil Malk Island) in the Republic of Palau were obtained by a diver from < 10 m water depth in October 2001 (Kawagata et al., 2005a, b). The East China Sea sediment was obtained in August 2016 during the NS16-442 cruise of the T/S Nagasaki Maru.
2.2 Extraction method
The method for extracting calcareous and siliceous components from diatom-bearing outcrop sediments, a calcareous layer from a marine lake core, and living materials is basically the same, although in the case of the latter, the kerosene step (2) is omitted. Kerosene is used to infiltrate the gaps inside diatomite or other older sediments; however, most modern sediment samples are still wet, so the kerosene step is not appropriate because kerosene is immiscible in water. The method described below can be seen in the flow chart presented in Fig. 1.
-
Approximately 1 g of sample is placed in a 50 cc screw-cap centrifuge tube (as it is easier to concentrate the material at the bottom of the tube) and the material is allowed to dry at 50 ∘C for about 2 h. If the sediment or sample is still hard, it is recommended to crush it using a mortar and pestle (e.g., Nishida, 1969).
-
An equal volume of kerosene is then added and allowed to soak into the sample for about 2 h. The kerosene is then removed and can be recycled if the kerosene is filtered using a 0.2 µm pore size filter paper.
-
An equal volume of highly condensed kitchen drain cleaning fluid (e.g., Look Noko (Strong and Effective) Pipeman made by LION – hereafter shortened to Look Pipeman; http://www.lion.co.jp/en/products/html/pro_c041.htm) is then added to the sample along with 3 times the volume of distilled water. The Look Pipeman cleaning fluid contains a total concentration of 0.8 % sodium hypochlorite (a powerful oxidizing agent), as well as sodium hydroxide (sometimes called lye or caustic soda, which dissolves organics and controls the pH), alkyl amine oxide (a surfactant), potassium salts of fatty acids (a soap and pesticide), and 2-phenoxyethanol (an antiseptic).
-
The centrifuge tube is then placed in a warm water bath maintained at 50 ∘C because the warm temperature accelerates the chemical reaction and helps to reduce the amount of chemicals and processing time.
-
After the particles have settled to the bottom of the centrifuge tube, the clear fluid at the top of the tube can be discarded and replaced by the same volume of distilled water. This washing step is repeated a minimum of five times. The whole process is finished when no more bubbles appear after shaking the tube. Sometimes the supernatant develops very slowly; thus, it may be necessary to use a centrifuge to reduce the working time.
The pellet at the bottom of the centrifuge tube may include both calcareous and siliceous components, as well as clay minerals and volcanic ash particles. However, centrifugation is often the best way to remove clays (Lentfer and Boyd, 1999), although use of detergents is another well-known method (e.g., sodium pyrophosphate in Bates et al., 1978) – here, both centrifugation and detergent (a constituent of Look Pipeman) are used. If the extraction process goes smoothly, then the total time for this procedure is about 5–12 h. Living materials can be extracted much more quickly (i.e., no kerosene step) and sometimes a smaller amount of sample can be used. For instance, if < 0.2 g of holothurian skin is placed in the centrifuge tube, the mean extraction time will be < 1 min. However, in general, it takes a minimum of 5 min to a maximum of 1 day.
2.3 Sample observation
After the extraction, permanent mounts using Mountmedia (Wako Pure Chemical Industries, Ltd.) or temporary mounts using microscopic immersion oil (IMMOIL-50CC, Olympus) were prepared and the samples were checked with a phase contrast objective lens on an Olympus BX40 light microscope, and photographed using an NY-1S relay lens (Micronet, Co., Ltd.) and a Canon EOS Kiss X6i camera. When necessary, samples were also observed in a JEOL JSM-6510LV scanning electron microscope (SEM).
3.1 Observations on treated and untreated fossil materials
Plate S1 in the Supplement shows the condition of the untreated samples in water; see Pl. S1, figs. 1a–b and 2a–b for the results from the diatom-bearing sediments of Mors and Oamaru, respectively, and fig. 3a–b for the JFL sediments.
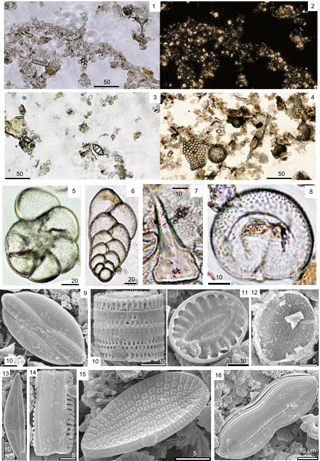
Plate 1Photomicrographs of the water mounts of the treated material. (1, 3) Mors diatomite, open nicols. (2) Mors diatomite, cross nicols. Many coccoliths were observed in cross nicols. (4) Oamaru diatom-bearing sediment. A centric diatom (Stephanopyxis sp.) and silicoflagellate (Naviculopsis sp.) can be seen in (4). (5–8) JFL sediments. (5) Ammonia sp. (foraminifer). (6) Biserial type of foraminifer. (7) Ichthyolith. (8) Presumably a juvenile foraminifer. (9–16) Diatoms found in the intestine of Holothuria atra from Okinawa. (9) Lyrella sp. (10) Paralia sp. (11) Surirella sp. (12) Unidentified diatom. (13) Amphora sp. (14) Unidentified araphid. (15) Cocconeis sp. (16) Nitzschia sp.
The treated material from the Mors diatomite (see Pl. 1, fig. 1, and figs. 2 and 3 for open and polarized light micrographs, respectively) revealed the presence of both calcareous (coccoliths) and siliceous (diatom and silicoflagellate fragments) microfossils, while the treated material from the diatom-bearing sediment from Oamaru (see Pl. 1, fig. 4) possessed diatoms and silicoflagellates. The microfossils in the JFL sediment are shown in Pl. 1, figs. 5–8: several foraminifera (Pl. 1, figs. 5–6), an ichthyolith (Pl. 1, fig. 7), and an unidentified calcareous microfossil, probably a juvenile foraminifer (Pl. 1, fig. 8).
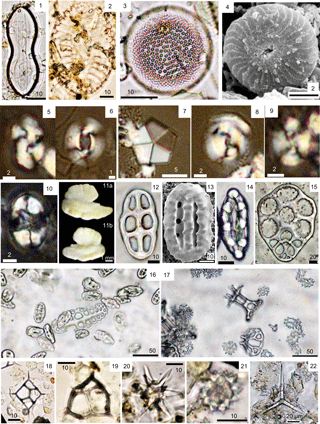
Plate 2Observations on smear slides of treated living material. (1–3) Diatoms in the intestine of H. forskali from the western Mediterranean Sea. (1) Diploneis sp. (2) Surirella sp. (3) Unidentified centric. (4, 6–8) Coccoliths in the intestine of H. atra from Okinawa. (4) Calcidiscus tropicus. (6) Reticulofenestra pseudoumbilicus. (7) Braarudosphaera bigelowii. (8) Coccolithus pelagicus. (5, 9–10) Coccoliths in the intestine of H. forskali from the western Mediterranean Sea. (5) Reticulofenestra perplexa. (9) Watznaueria cf. biporta. (10) Eifellithus cf. turriseifellii. (11a–b) Otoliths of the sailfin sandfish (Arctoscopus japonicus) from Japanese waters. (12–17) Holothurian ossicles. (12) Holothuria hilla from Bohol. (13) H. tubulosa from the Mediterranean Sea. (14) H. polii from the Mediterranean Sea. (15) A member of the Synaptidae from Guam. (16) H. polii from the Mediterranean Sea. (16) H. atra from Okinawa. (18–22) Microfossils from the intestine of H. forskali from the western Mediterranean Sea. (18) Dictyocha sp. (19) Corbisema sp. (20–22) Sponge spicules.
3.2 Extraction from living material
The diatoms in Pl. 1, figs. 9–16, taken with a SEM, were extracted from the intestine of the holothurian Holothuria atra from Okinawa. In addition, the diatoms from Pl. 2, figs. 1–3 were found in the intestine of the holothurian H. forskali from the Mediterranean Sea. The coccoliths from Pl. 2, figs. 4–10 were also found in the H. atra intestine. Otoliths of the sailfin sandfish (Arctoscopus japonicus), caught off the Shonai and Karo coasts, are shown in Pl. 2, fig. 11a–b, respectively. Plate 2, figs. 12–17 are modern holothurian ossicles, sampled from Bohol, the Mediterranean Sea, and Guam. The mineralized components shown in Pl. 2, figs. 18–22 were found in the intestine of the holothurian H. forskali, and include silicoflagellates (Pl. 2, figs. 18–19) and sponge spicules (Pl. 2, figs. 20–22).
Plate S2 shows the step-by-step situation during the washing process of East China Sea sediment (Pl. S2, figs. 1a–f) and Holothuria leucospilota ossicles from an animal collected at Taketomi Island (Pl. S2, fig. 2a–f). Plate S2 also includes the photomicrographs taken of the discarded supernatant part (Pl. S2, fig. 1b′–e′ for the sediment and Pl. S2, fig. 2b′–e′ for the ossicles) and the remaining pellet (Pl. S2, fig. 1f′ for the sediment and Pl. S2, fig. 2f′ for the ossicles). The supernatant did not contain any microfossils or ossicles. This is likely due to the difference in specific weight between the kitchen drain cleaner and the microfossils and ossicles, with the kitchen drain cleaner being the lighter one. In the case of the sediment, the supernatant part can be seen in Pl. S2, fig. 1c–e, first reaching the > 20 mL mark (Pl. S2, fig. 1c) and finally the > 30 mL mark (Pl. S2, fig. 1e) of the centrifuge tube. Plate S2, fig. 1e represents a time close to the termination of the washing process. In the case of the holothurian, the skin is removed during the washing process between the stages shown in Pl. S2, fig. 2c and d after termination of the bubbling.
Figure 1 shows the differences between the methods of Nishida (1969), Nagumo (1995), Oda and Sato (2013), and this study. However, none of them are useful for extracting microfossils from hard rocks (e.g., dolomite, chert, shale), which require other methods. This pipe cleaner method has already been tested using the JFL sediment and sediment that includes land plant debris, with the dissolution of the latter or other organics proceeding very smoothly. The improved extraction method outlined herein will be useful for the rapid processing of a wide range of materials, both living and fossil. It involves two main chemicals, kerosene and kitchen drain cleaner fluid, both of which are cheap, easy to obtain, and easy to discard afterwards. Kerosene, used here as a substitute for naphtha in the Oda and Sato method (2013), is a familiar fuel oil for room heaters or hot water supply systems in Japan, while the kitchen drain cleaning fluid can be bought at a reasonable cost at supermarkets, drug stores, or do-it-yourself or daily grocery shops in Japan. Similar nonacidic products are also available in other countries. However, if acidic products are used the calcareous microfossils including coccoliths will be dissolved, so care must be taken when choosing the kitchen drain cleaner fluid.
-
The improved method using kerosene and/or kitchen drain cleaner liquid is a useful procedure for studying biogenic siliceous and calcareous materials (e.g., coccoliths, silicoflagellates, diatoms, sponge spicules, holothurian ossicles, foraminifers, and fish otoliths) in various types of sample.
-
The advantage of this method is that both the calcareous and siliceous components can be cleaned up at the same time, without noticeable dissolution or etching.
-
This method can reduce the total cleaning time to minutes or hours instead of days, seemingly without any demerit, and involves neither any dangerous or expensive chemicals nor the need for special safety measures for discarding waste chemicals. The sample and chemical volumes can also be reduced.
All the data in this project are shown herein or in the Supplement.
The supplement related to this article is available online at: https://doi.org/10.5194/jm-37-249-2018-supplement.
The authors declare that they have no conflict of interest.
We would like to thank the following people for providing the material used
in this study. The holothurian samples from the Mediterranean Sea and Guam
were sent to us by Catherine Riaux-Gobin and Guillerme Iwankow (University of
Perpignan/CNRS, France) and by Alex Kerr (University of Guam), respectively.
The diatom-bearing samples from Mors and Oamaru were provided by Emma Sheldon
(Geological Survey of Denmark and Greenland) and Jakub Witkowski (Natural
Sciences Education and Research Centre, University of Szczecin),
respectively. The sediment sample of East China Sea was taken by
Yasuhiro Morii (ship captain) and associate Nobuhiro Yamawaki (chief officer)
of the T/S Nagasaki Maru, which belongs to the Faculty of Fisheries, Nagasaki
University. The holothurian sampling in Okinawa was coordinated by
Ikuko Uezato and Yuki Uezato. Hisako Kudo and Hiroko Tsutsui acquired the
sailfin sandfish from Shonai and Karo, respectively. The SEM microphotographs
were taken by Ryohei Fujita, a past master course student in our laboratory.
The paper benefited from the constructive comments of the two reviewers,
Xavier Crosta and Jakub Witkowski, as well as of the journal editor,
Taniel Danelian.
Edited by:
Taniel Danelian
Reviewed by:
Xavier Crosta and Jakub Witkowski
Abrantes, F., Gil, I., Lopes, C., and Castro, M.: Quantitative diatom analyses – a faster cleaning procedure, Deep-Sea Res. Pt. I, 52, 189–198, 2005.
Bates, C. D., Coxon, P., and Gibbard, P. L.: A new method for the preparation of clay-rich sediment samples for palynological investigation, New Phytol., 81, 459–463, 1978.
Bourdon, M.: Méthode de dégagement des microfossiles par acétolyse à chaud, Compte rendu sommaire des séances de la Société géologique de France, 267–268, 1962.
Chambers, L. M., Pringle, M., Fitton, G., Larsen, L. M., Pedersen, A. K., and Parrish, R.: Recalibration of the Paleocene-Eocene boundary (P-E) using high precision U-Pb and Ar-Ar isotopic dating, Abstract, EGS-AGU-EUG Joint Assembly Nice, 2003.
Collins, J. S. H., Schulz, B. P., and Jabosen, S. L.: First record of brachyuran decapods (Crustacea, Decapoda) from Fur Formation (early Eocene) of Mors and Fur Island, Denmark, Bulletin of the Mizunami Fossil Museum, 32, 17–22, 2005.
Faegri, K., Kaland, P. E., and Krzywinski, K.: Textbook of pollen analysis, IV Edn., Botanical Institute, University of Bergen, Norway, John Wiley and Sons, Co., Ltd., 1989.
Fire Service Act: Act No. 186 of 1948, amendment in Act 41 of 2008, translated into English on the Ministry of Justice, Japan, available at: http://www.japaneselawtranslation.go.jp/law/detail/?printID=&re=02&id=1994&lvm=01&vm=02 (last access: 31 January 2018), 2008.
Franke, A.: Die Präparation von Foraminiferen und anderen mikroskopischen Tierresten, in: Lehrbuch der praktischen Geologie, Mineralogie, und Palaeontologie, edited by: Keilhack, K., 4th Edn., Enke-Verlag, Stuttgart, 509–533, 1922.
Hanken, N. M.: The use of sodium tetraphenylborate and sodium chloride in the extraction of fossils from shales, J. Paleontol., 53, 738–740, 1979.
Ikeya, N. and Chinzei, K.: Microfossils, 2nd Edn., translated by: Armstrong, H. A. and Brasier, M. D., Asakura Publishing Co., Ltd., 276 pp., 2007 (in Japanese).
Jarochowska, E., Tonarová, P., Munnecke, A., Ferrová, L., Sklenř, J., and Vodrážková, S.: An acid-free method of microfossil extraction from clay-rich lithologies using the surfactant Rewoquat, Palaeontol. Electron., 16, 16 pp., 2013.
Jørgensen, F., Sandersen, P. B. E., Auken, E., Lykke-Andersen, H., and Sørensen, K.: Contributions to the geological mapping of Mors, Denmark – A study based on a large-scale TEM survey, Bulletin of the Geological Society of Denmark, 52, 53–75, 2005.
Kawagata, S., Yamasaki, M., and Jordan, R. W.: Acarotrochus lobulatus, a new genus and species of shallow water benthic foraminifer from Mecherchar Jellyfish Lake, Palau, NW Equatorial Pacific Ocean, J. Foram. Res., 35, 44–49, 2005a.
Kawagata, S., Yamasaki, M., Genka, R., and Jordan, R. W.: Shallow-water benthic foraminifers from Mecherchar Jellyfish Lake (Ongerul Tketau Uet), Palau, Micronesica, 37, 215–233, 2005b.
Lee, D. E., Scholz, J., and Gordon, D. P.: Paleoecology of a late Eocene mobile rockground biota from North Otago, New Zealand, Palaios, 12, 568–571, 1997.
Lentfer, C. J. and Boyd, W. E.: An assessment of techniques for the deflocculation and removal of clays from sediments used in phytolith analysis, J. Archaeol. Sci., 26, 31–44, 1999.
Liang, D. and Readey, D. W.: Dissolution of crystalline and amorphous silica in hydrofluoric-hydrochloric acid mixtures, J. Am. Ceram. Soc., 70, 570–577, 1987.
Lirer, F.: A new technique for retrieving calcareous microfossils from lithified lime deposits, Micropaleontology, 46, 365–369, 2000.
Nagumo, T.: Simple and safe cleaning methods for diatom samples, Diatom, 10, 88, 1995 (in Japanese).
Nielsen, J. K. and Jakobsen, S. L.: Extraction of calcareous macrofossils from the upper Cretaceous white chalk and other sedimentary carbonates in Denmark and Sweden: The acid-hot water method and the water blasting technique, Palaeontol. Electron., 7, 11 pp., 2004.
Nishida, S.: Some observations on the microfossils by a new technique, Bulletin of the Nara University of Education, 17, 89–102, 1969 (in Japanese with English abstract).
Novitski, L. and Kociolek, P.: Preliminary light and scanning electron microscope observations of marine fossil Eunotia species with comments on the evolution of the genus Eunotia, Diatom Res., 20, 137–143, 2005.
Oda, M. and Sato, T.: New edition of the manual for microfossil researches, Asakura Publishing Co., Ltd., 110 pp., 2013 (in Japanese).
Remin, Z., Dubicka, Z., Kozlowska, A., and Kuchta, B.: A new method of rock disintegration and foraminiferal extraction with the use of liquid nitrogen [LN2]. Do conventional methods lead to biased paleoecological and paleoenvironmental interpretations?, Mar. Micropaleontol., 86–87, 11–14, 2012.
Shiono, M. and Jordan, R. W.: Recent diatoms of Lake Hibara, Fukushima Prefecture, Diatom, 11, 31–63, 1995.
World Health Organization: Essential medicines WHO model list, 19th Edn., 51 pp., 2015.
Yoshida, W., Onakatomi, T., and Ishida, S.: Relation between the growth of complex plate ossicle and the body length of sea cucumber Apostichopus japonicus, Bulletin of the Faculty of Agriculture and Life Science, Hirosaki Univ., 9, 15-2, 2006 (in Japanese).





