the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Taxonomic review of living planktonic foraminifera
Geert-Jan A. Brummer
Applications of fossil shells of planktonic foraminifera to decipher past environmental change and plankton evolution require a robust operational taxonomy. In this respect, extant planktonic foraminifera provide an opportunity for benchmarking the dominantly morphological species concepts and classification of the group by considering ecological, physiological and genetic characters. Although the basic framework of the taxonomy of extant planktonic foraminifera has been stable for half a century, many details have changed, not the least in light of genetic evidence. In this contribution, we review the current taxonomy of living planktonic foraminifera, presenting a comprehensive standard that emerged from the meetings and consultations of the SCOR/IGBP Working Group 138 “Planktonic foraminifera and ocean changes”. We present a comprehensive annotated list of 50 species and subspecies recognized among living planktonic foraminifera and evaluate their generic and suprageneric classification. As a result, we recommend replacing the commonly used names Globorotalia menardii by G. cultrata and Globorotalia theyeri by G. eastropacia, recognize Globorotaloides oveyi as a neglected but valid living species, and propose transferring the three extant species previously assigned to Tenuitella into a separate genus, Tenuitellita. We review the status of types and designate lectotypes for Globoturborotalita rubescens and Globigerinita uvula. We further provide an annotated list of synonyms and other names that have been applied previously to living planktonic foraminifera and outline the reasons for their exclusion. Finally, we provide recommendations on how the presented classification scheme should be used in operational taxonomy for the benefit of producing replicable and interoperable census counts.
- Article
(9580 KB) - Full-text XML
- Comment
-
Supplement
(386 KB) - BibTeX
- EndNote
Planktonic foraminifera are marine protists with ornate calcite shells, which have inhabited upper ocean waters since the Jurassic. They abound in all oceanic settings, ranging from oligotrophic tropical gyres and productive upwelling regions to the coldest waters of the polar regions (Bé, 1977). Whilst their biomass constitutes only a minor part of the plankton, their calcite shells form a significant portion of the pelagic carbonate flux (Schiebel, 2002). These shells accumulate in marine sediments, where they form a unique archive of climatic and biotic change. This archive can be deciphered because the elemental and isotopic composition of foraminiferal shells contains chemical and physical signals related to the state of the habitat in which they were precipitated (Henderson, 2002). This makes planktonic foraminifera an important tool in paleoceanography and paleoclimatology (Kucera, 2007). However, since the seasonal flux and the calcification depth differ among species, the application of fossil shells of planktonic foraminifera to decipher past environmental change requires species-specific analyses (Jonkers and Kucera, 2017) and is thus contingent on the existence of robust operational taxonomy.
Planktonic foraminifera were initially discovered and studied in sediment samples. Using the richness of morphological characters of their shells, their classification was based entirely on the characters of their skeleton. In this way, living and fossil species concepts are congruent, and the evolutionary origin of living species can be traced in the fossil record (e.g. Aze et al., 2011). In the past two decades, advances in molecular genetics provided an opportunity to benchmark the classical morphological species concept by analyses of DNA sequence divergence. This new and independent information had two consequences for taxonomy. First, it largely confirmed the choice and interpretation of traits used in species concepts based solely on shell morphology, resulting only in minor amendments (Darling et al., 2006; Aurahs et al., 2011; Weiner et al., 2015; Morard et al., 2019a). Second, it led to the discovery of extensive genetic diversity within most morphologically defined species, which likely signifies the presence of biological (reproductively isolated) species that are morphologically similar or even indistinguishable, i.e. cryptic species (Darling and Wade, 2008).
In this contribution, we review the current taxonomy of living planktonic foraminifera, with the aim to introduce the comprehensive standard that emerged from the meetings and consultations of the SCOR Working Group 138 “Planktonic foraminifera and ocean changes” (Ganssen and Kucera, 2012). In addition to presenting an annotated list of living species, their generic classification and a review of types, we provide an annotated list of synonyms and other names that have been applied previously but are here considered inappropriate for the use on living planktonic foraminifera. It is not our intention to revise taxonomic descriptions or to provide differential diagnoses or new illustrations apart from the lectotypes designated herein. For these aspects of the taxonomy, the reader is referred to the many existing manuals and classical taxonomic works (Parker, 1962; Saito et al., 1981; Kennett and Srinivasan, 1983; Schiebel and Hemleben, 2017). Instead, our primary intention is to explain the current concepts and (supra-)generic assignment of extant species, justifying the retention or rejection of various taxa and names for better understanding of the diversity of the group through time.
We begin by briefly commenting on the name of the object of this review, explaining the chosen usage of “planktonic” and “foraminifera”. The recommendation that we present is based on a pragmatic approach of conserving the most common usage. We do not wish to initiate a detailed linguistic debate nor do we intend to repeat arguments presented at length in the literature, so the justifications are left in their simplest form. With respect to the adjective “planktonic”, a second version “planktic” has also been in use. A remarkable body of literature exists advocating each version, using arguments that all seem legitimate (Burckhardt, 1920; Rodhe, 1974; Hutchinson, 1974; Martinsson, 1975, 1979, 1982; Teichert, 1981; Emiliani, 1991a, b). Notwithstanding this debate, the current (year 2021) usage favours “planktonic” by more than an order of magnitude (based on a search in Google or more specifically on Google Scholar). A higher popularity also holds for the combination “planktonic foraminifera”. We also note that this distinction has been consistent for over a decade, because the same pattern of usage was noted by Pearson (2012). Therefore, we recommend retaining the adjective “planktonic”. This does not mean that we object to the usage of the less popular form, but in the absence of a grammatical consensus, we plead with the community to conserve the more common usage. A similar issue concerns the usage and grammar of “foraminifera”. This topic has been exhaustively treated by Lipps et al. (2011). Following that study, we recommend the following usage: capitalized, the word “Foraminifera” is Latin and refers to a taxonomic unit; not capitalized, the word is vernacular and English and can be both singular and plural (like “sheep”), referring to one or more specimens or taxa. Like Lipps et al. (2011), we see no reason to endorse the seemingly “more” English, but far less used, substantive “foraminifer” with its plural form “foraminifers”.
Although the oldest illustrations of their shells date to the 18th century (e.g. Soldani, 1791), planktonic foraminifera were first systematically described by Alcide Dessalines d'Orbigny, who is the author of the earliest validly named extant species (d'Orbigny, 1826). The basis of the modern taxonomy has been set in the second half of the 19th century in connection with the exploration of the ocean interior and the deep sea by British and German expeditions. The current classification is the result of the advent of deep-sea drilling and paleoceanography a century later (Fig. 1). A milestone in the development of the current classification is the study by Parker (1962), whose scheme was adopted by Bé (1967a) for studies on living plankton and by the CLIMAP project (1976) to serve as a basis for paleoecological reconstructions by means of geochemical proxies and assemblage census counts in Quaternary sediments. This scheme has prevailed for decades without major amendments, indicating that it succeeded in covering a large part of the diversity of living planktonic foraminifera. Indeed, new species described after Parker (1962) comprise mainly small and (apparently) rare forms. At that time, the higher-level classification of living and fossil planktonic foraminifera began to converge on the current convention, with higher-rank taxa reflecting differences in shell wall texture (Steineck and Fleisher, 1978) and ontogenetic morphology (Brummer et al., 1986), i.e. processes related to biomineralization, metabolism and growth, with genera being defined by differences in shell architecture and aperture modifications (e.g. Loeblich and Tappan, 1984). This choice of characters and their phylogenetic interpretation (Olsson et al., 1999; Pearson et al., 2006; Wade et al., 2018a) has been largely vindicated by genetic data generated in the last two decades (e.g. Aurahs et al., 2009; Morard et al., 2019b).
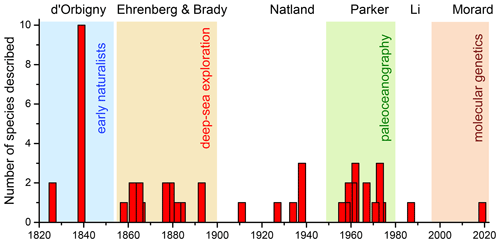
Figure 1Timeline of the year of original description of 50 extant planktonic foraminifera species and subspecies accepted in this study (including the subspecies G. ruber albus described in 2019), highlighting the main “eras” of taxonomy, with names of representative authors.
Because of its geological “roots”, the current classification of planktonic foraminifera is based on fully grown specimens, as are typically found in sediment samples. In contrast, specimens found in the living plankton are dominated by maturing forms, which often lack the distinctively shaped final chambers (e.g. the spherical chamber in Orbulina universa) and terminal morphological features such as a bulla (e.g. in Globigerinita glutinata) or crust (e.g. in Pulleniatina obliquiloculata). This reflects the life cycle of planktonic foraminifera, in which the formation of distinctive final chambers and other morphological features ends further shell growth and marks reproductive maturity (terminal stage), after which the shell settles on the seafloor (Brummer et al., 1986). Further complicating conventional taxonomy is the fact that the ontogeny of planktonic foraminifera is associated with substantial transformation of shell architecture, such as in Trilobatus sacculifer, and a late ontogenetic emergence of taxonomically important architectural elements, such as chamber elongation, or apertural modifications, such as the presence of supplementary apertures (Brummer et al., 1986). As a result, separate taxa have been proposed for immature and mature specimens of the same species, both in the living plankton and the sediment. Since the transformation of shell morphology through ontogeny is preserved in the early part of the shell, this issue is now largely resolved. However, a lack of diagnostic characters in maturing specimens in the plankton often precludes their species or even genus-level identification, often necessitating (and indeed justifying) the use of open nomenclature.
Taxonomy is a communication system which is shared by scientists of different disciplines and which is based on a wide range of data types. In the case of the extant planktonic foraminifera, the involved data sources include morphology, molecular genetics and species ranges observed in the fossil record, and the users include biologists, biostratigraphers, geochemists and paleoceanographers. Inevitably, there are differences in priority and emphasis between these groups, but having a common taxonomy is immensely valuable. This is only possible with compromise, and this has been the guiding principle of our taxonomic work. The most obvious case, where a conflict arises and compromise is required, is the different usage of species concepts between biologists and biostratigraphers. In biostratigraphy, emphasis is on the emergence and demise of a certain morphology, which needs a formal label. Such a typological approach is useful in biostratigraphic practice, but it cannot be transferred on extant taxa, whose taxonomic identity can be confirmed by independent, genetic evidence and which must represent evolutionarily significant units. Conversely, species concepts circumscribed strictly by genetic evidence or defined by discontinuity in morphospace (morphospecies) cannot be easily transferred in the fossil record in the presence of anagenetic change (as noted and explained in detail by Aze et al., 2011) and fail to stabilize by name many biostratigraphically useful morphologies. Being confronted with the task of classifying extant taxa, we here must adhere to the biological understanding of a species, but we do so without claiming that such a system must be transferred onto the fossil record. Instead, we highlight key cases where names that must be rejected for extant taxa may be retained when classifying fossil species. This problem is illustrated by cases like the usage in fossil and extant material of Trilobatus trilobus and T. sacculifer (Spezzaferri et al., 2015) or the nature of the fossil taxon Pulleniatina finalis, which describes a morphology that ranges to the present, but whose existence as a biological taxon cannot be substantiated (Pearson and Penny, 2021).
What we cannot endorse is a continued formal taxonomic treatment of morphological variants, referring to a subset of specimens within the range of variability of the constituent taxon without evolutionary or biostratigraphic significance, or any artificial taxa referring to adventitious characters, aberrations or separate names for adult and pre-adult specimens of the same taxon. Having said that, we have to admit that in the fossil record it is not always possible to determine which characters only represent facultative ontogenetic developments or aberrations and which do not. This means that in the fossil record, we will always be left with artificial taxa, creating obstacles to a full unification of the taxonomy of fossil and extant species.
With respect to taxa beyond the rank of species, we believe that the spirit of compromise is served best by accepting taxa of higher rank, such as genera, that are paraphyletic, i.e. which do not include all descendants of the nearest common ancestor. Whilst we should strive, both for extant and fossil material, to create and use monophyletic taxa, we believe it is impossible and impractical to ensure strict monophyly at all taxonomic levels. Paraphyletic taxa arise because morphological innovations used to circumscribe genera and higher-rank taxa arise within single lineages, with their surviving sister taxa retaining the ancestral morphology. Indeed, in the notable case of G. siphonifera, even the species concept is paraphyletic, because the named sister taxa G. radians and G. calida clearly originate from different lineages within the genetically diverse but otherwise morphologically homogenous G. siphonifera (Weiner et al., 2015).
3.1 Species classification
At the species level, our taxonomy includes 48 holoplanktonic species and subspecies, which are phylogenetically coherent (monophyletic or paraphyletic) and are known to occur alive in the plankton. In addition to the 48 holoplanktonic taxa, we also include two forms with serially arranged chambers, which live in the plankton but may not follow a holoplanktonic lifestyle. The scheme builds on the classification as presented in Hemleben et al. (1989) who recognized 43 holoplanktonic species, with Neogloboquadrina incompta, Globigerinoides elongatus and Globigerinella radians being added later based on genetic confirmation of their different morphologies (Darling et al., 2006; Aurahs et al., 2011; Weiner et al., 2015) and accepted by Schiebel and Hemleben (2017). Recently, Globigerinoides ruber albus was established as a subspecies name necessary to differentiate the two genetically distinct lineages within G. ruber (Morard et al., 2019a). Finally, we reviewed all species names known to us that were established for living foraminifera collected from the plankton. As a result, next to the elusive Globorotalia cavernula, which we provisionally retain, we here also recognize the species Globorotaloides oveyi. With respect to the names used for the accepted taxa, unlike Schiebel and Hemleben (2017), we retain T. fleisheri and reject T. compressa as an invalid synonym. Like Hemleben et al. (1989) and Schiebel and Hemleben (2017), and in line with André et al. (2013) we prefer the use of “sacculifer” as the name for the morphologically variable but genetically homogenous single living species of the genus Trilobatus (Spezzaferri et al., 2015). In our research on the history of classification we came to the conclusion that the name “menardii” has been used incorrectly for the extant taxon, whose appropriate name must be Globorotalia cultrata. We also discovered that Globorotalia theyeri must be considered a junior synonym of G. eastropacia.
Next to the inventory of accepted taxa, we provide a list of species names that have been used previously in association with living planktonic foraminifera or with fossil material from the late Quaternary, but which are here excluded (Table 3). We provide an opinion for each name in terms of its likely (or certain) counterpart in our classification and the reason why it is not recognized as appropriate for the classification of living planktonic foraminifera. In constructing the list, we made use of the comprehensive review of species-level names by Saito et al. (1981), including the numerous names proposed by McCulloch (1977). We also considered the names of species ranging to the present as presented by Aze et al. (2011) and the synonyms highlighted by Siccha and Kucera (2017) as well as names listed in internet inventories (Hayward et al., 2020; Young et al., 2020; Huber et al., 2016). Our decisions are based on observations of type material as well as cross-referencing with original (re)descriptions and major monographs. Although we do not formally establish new taxa or names in this review, this work has been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID alphanumeric component to the prefix http://zoobank.org/. The LSID for this publication is urn:lsid:zoobank.org:pub:9864F738-9F9C-482B-8EEA-D1ED4B189B5D.
An extensive list of other species names for possibly living taxa, most of them proposed in the 19th century by Ehrenberg but rarely (or never) used since, is given in Saito et al. (1981, pp. 16–18). Apart from the 12 nomina nuda (originally listed without description or illustration), which are taxonomically neither valid nor available, another 66 names are given many of which should be regarded as nomina dubia, e.g. of which the type material is lost and without a description and/or illustration that would allow them to be identified as conspecific with any taxa described later. Some of these names may well prove useful in future revisions, in the manner of G. radians that was reinstated by Weiner et al. (2015); others are likely lost to science. Even if some of the names could represent senior synonyms, most names have not been used by any later authors (except in listings such as Saito's et al., 1981), so that probably all later proposed species names should effectively be considered as nomina conservanda against the names listed by Saito et al. (1981). Therefore, most of the Ehrenberg's names listed on pp. 16–18 of Saito et al. (1981) are not included here. Similarly, we have systematically avoided new names (including generic and suprageneric) proposed in Fordham (1986), as this publication does not follow the principles of binomial nomenclature and the names described therein should thus be regarded as unavailable (see Loeblich and Tappan, 1988; Haman, 1988).
In general, we identify four reasons why a species name has not been accepted by us for living planktonic foraminifera:
- a.
name referring to a morphological variant within an accepted species, whose taxonomic status as a species is in doubt (example: Biorbulina bilobata used for an aberrant form of Orbulina universa);
- b.
junior synonym of a valid species (example: Globigerinella aequilateralis for G. siphonifera);
- c.
invalid (for a formal reason) or suppressed name for a valid species (example: Tenuitella compressa for T. fleisheri);
- d.
name referring to an extinct species, erroneously applied to a valid extant species (example: Neogloboquadrina humerosa used for N. dutertrei) or to a morphological variant of a valid extant species (example: Orbulina suturalis used for an aberrant form of living Orbulina universa).
Among the 128 names listed (Table 3), 51 are considered synonymous, 24 refer to extinct taxa, and 10 are invalid or informal names. The remaining 42 names refer to morphological variants within accepted species, and we note that a significant number of such names may prove useful in future revisions, since the morphological distinction associated with this name, here considered as taxonomically not significant, may later prove to be associated with consistent genetic divergence. Indeed, cryptic genetic diversity is prevalent among planktonic foraminifera (Darling and Wade, 2008), and many of the involved morphospecies are known to harbour substantial and systematic phenotypic variability.
3.2 Genus-level classification
The generic classification of living planktonic foraminifera (Fig. 2) follows a convention where genera are established to demarcate a significant difference in shell architecture (Olsson et al., 1999; Pearson et al., 2006; Wade et al., 2018a). Such demarcations are often present in single lineages, which may contain several fossil species but are represented by a sole survivor. As a result, the classification of modern planktonic foraminifera appears to be cluttered with monotypic genera (genera containing only one species). To remain consistent with the fossil taxonomy, we here retain most of the commonly used monotypic genera, including all those which comprise additional fossil species (such as Beella, Globoquadrina, Globoturborotalita, Orbulina or Pulleniatina). Unlike Schiebel and Hemleben (2017), our classification does not recognize Bolliella, which is here subsumed under Globigerinella. This is because Bolliella has always been strictly monotypic and defined by a trait that is recurrent in the clade and thus does not constitute a large and singular transition in shell architecture needed to justify its own genus. Nevertheless, we stress that Bolliella would be monophyletic and the remaining Globigerinella paraphyletic, and should the use of Bolliella prove useful to elucidate the diversity of fossil or living pseudocryptic taxa, the name may be reinstated.
We retain the monotypic Hastigerinella alongside Hastigerina, originally established to account for the large change in chamber architecture associated with the bifurcating digitate chambers of Hastigerinella. The retention of Hastigerinella is essential to avoid confusion in assigning the species name “digitata” to Hastigerina, because the same combination has been wrongly used in the past for the species that is now classified as Beella digitata. The genus Hastigerinella has been subject to formal ICZN rulings that were necessary to stabilize the nomenclature of fossil taxa (Coxall, 2003: ICZN Case 3245; ICZN Opinion 2105, 2005). Similarly, the application of a phylogenetically consistent taxon concept at the level of genera leads us to accept the genus Trilobatus, which is necessary to avoid a polyphyletic Globigerinoides (Spezzaferri et al., 2015), and following Morard et al. (2019a), we return the species “tenella” to Globigerinoides, where it was placed originally by Parker (1962). In addition, we note that the name Tenuitella, commonly applied to three distinct extant small microperforate taxa with extraumbilical apertures, is typified by the latest Eocene to late Oligocene species Globorotalia gemma, and there is at present no consensus on how the extant taxa are related to the Paleogene and early Neogene representatives of this group (Pearson et al., 2018). Genetic data indicate that the extant taxa are relatively recently derived from Globigerinita (Morard et al., 2019b), and there exists a conspicuous Messinian–Zanclean gap between the known range of the extant taxa and the extinction of T. clemenciae as the sole Neogene survivor of the Paleogene group (e.g. Kennett and Srinivasan, 1983). Therefore, we here propose to use Tenuitellita for the clade comprising the three extant species. This is because Tenuitellita Li, 1987, is typified by Globigerinita iota Parker, 1962, and there is thus no doubt that this validly established genus name is referring to the extant clade (Haman, 1988).
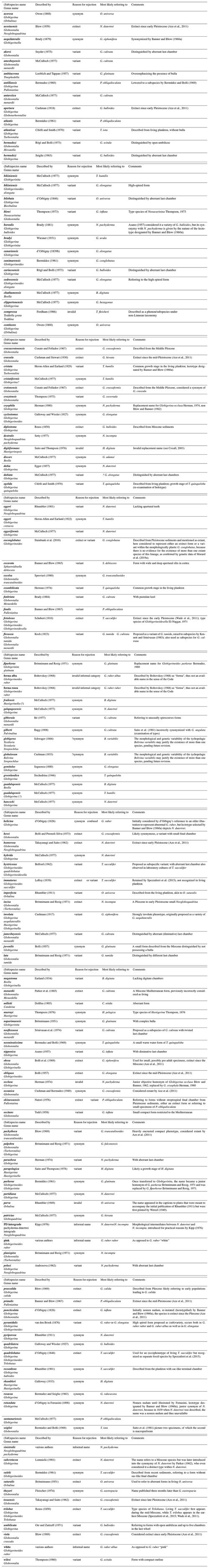
Like Schiebel and Hemleben (2017) and most other workers, we retain a broad concept of Globorotalia, but we highlight here that the existing genus level names in this clade such as Globoconella, Truncorotalia, Menardella and Hirsutella (Table 1) are phylogenetically consistent and their use may have merit especially when considering the taxonomy of extinct lineages. However, should any of these names be reinstated in future revisions, we caution about the complexities involved with homonymy and synonymy issues associated with these names as detailed in Table 1. Finally, four monotypic genera are retained because the taxonomic position of the involved species is unclear, precluding their assignment to other existing genera. This refers to the spinose Orcadia, as well as the non-spinose Dentigloborotalia and Berggrenia and the triserial Neogallitellia. Their morphology differs from other planktonic foraminifera in unique ways,, and their fossil record is as yet too poorly documented to reconstruct their ancestry. As a result, the phylogenetic assignment of these four species cannot be resolved at present, and they must remain assigned to their idiosyncratic genera.
3.3 Suprageneric classification
The suprageneric classification adopted in this study (Fig. 2) follows a phylogenetically consistent concept in which we assign all extant planktonic foraminifera to the order Rotaliida but no longer assign the microperforate planktonic foraminifera to the same suborder as the macroperforate globigerinids and globorotaliids. Instead, we assign the three clades to different superfamilies. This distinction accounts for the possibility of separate origin of each clade from different benthic ancestors (Morard et al., 2019b). At the same time, should it transpire that some of the clades share a common ancestor, the superfamilies will remain valid, albeit at the cost of one of them, or their common ancestor, becoming paraphyletic. Unlike in the recent revision presented in the Atlas of Oligocene Planktonic Foraminifera (Pearson et al., 2018), we retain Candeina among the microperforate clade. This is consistent with its microperforate wall structure (Steineck and Fleisher, 1978) and early ontogeny (Brummer, 1988a), as well as with genetic evidence for its close link with Globigerinita (Ujiié and Lipps, 2009; Morard et al., 2019b). As a result, adhering to the principle of priority, we must classify the microperforate clade following Cushman (1927) as Candeinidae rather than using the name Globigerinitidae after Bermúdez (1961), which was adopted by Pearson et al. (2018). By retaining the Candeinidae, whilst separating the microperforate clade from the macroperforate foraminifera, Cushman's (1927) taxon must be raised to superfamily level as Candeinoidea n. transl., with Cushman (1927) remaining the author.
Overall, the classification of genera follows the scheme as implemented in Hemleben et al. (1989) and Schiebel and Hemleben (2017), with the exception of Orcadia, which is here returned to the family Hastigerinidae, following its original assignment by Rögl and Bolli (1973). The phylogenetic position of this genus has always been enigmatic, and in the absence of independent (genetic) evidence, we must continue to consider its classification as provisional. Finally, we note that Fordham (1986) erected a host of apparently suprageneric clade names, which are not reproduced here, because they are considered taxonomically invalid, as the work in which they appear did not follow the principles of binomial nomenclature. Loeblich and Tappan (1988, pp. 717–718) give a list of those names, noting “The following names were proposed as “cladegroups”, a category not recognized under the International Code on Zoological Nomenclature (henceforth abbreviated as “Code”), hence not available.”.
3.4 Status of types
For all accepted species, we provide information on the status of the type material as the primary reference fixing the taxonomy to a physical specimen (Table 2, Fig. 3). The existence of suitable types for the vast majority of the extant species is due to the monumental effort of Banner and Blow (1960a and subsequent work), who revised many of the species that had been described at times when types were not designated (Table 2). Our review shows that only six of the existing types originate from the living plankton and are thus unambiguously referable to living species. The majority of the types are derived from sediments, mainly from seafloor deposits of Holocene (surface sediment) or Pleistocene age (down-core). Two types of d'Orbigny (G. bulloides and G. elongatus) are from beach sediments from Rimini in Italy, with uncertain age, because the sediments contain reworked Miocene fauna (Lamb and Beard, 1972). The types are by most authors considered Quaternary (e.g. Spezzaferri et al., 2018b), and in both cases the concept of the taxa is not contested. In two cases, the types are derived from Neogene sediments, requiring a careful evaluation to prove that the fossil types refer to the same taxon as the living species. Indeed, the designation of types based on fossil material caused instability in nomenclature, as shown by the unfortunate case of G. menardii, which led to decades of confusing and inconsistent usage. We fear that, having fossil types of uncertain status, Globigerina falconensis and Globoquadrina conglomerata are likely to follow suit.
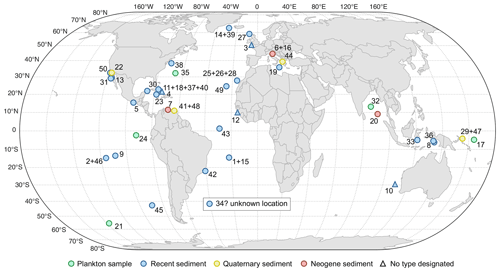
Figure 3Location and nature of material from which types of extant species of planktonic foraminifera have been derived. Numbers refer to species as listed in Table 2. Triangles refer to the origin of type material in instances where no type has been formally designated. Question mark indicates an instance where the type locality could not be identified precisely.
Table 2Status of types of all species of living planktonic foraminifera recognized in this study. Species are listed in the same order as in the annotated list in the text, and the localities are shown in Fig. 3.
a Type localities where coordinates are estimated from maps or descriptions, b type localities which cannot be traced to a specific location, c where no type has been designated, we list localities mentioned in the original descriptions of the species.
Six species were found to have no designated types. Among these, we succeeded in locating the original type material for G. uvula and pertinent material for G. rubescens, allowing us to designate types for these species (Figs. 4, 5). No types exist for C. nitida, G. glutinata and G. radians. The original material on which the species descriptions were based is in all cases considered lost. However, the localities of the original material can be narrowed down to specific regions, and all species were said to originate from recent sediments. In all three cases, the species concepts are not in doubt, and we therefore leave the designation of neotypes to future revisions. Finally, no type appears to have been designated for B. variabilis. Whilst suitable topotypic (or even syntypic) material is likely available, we refrain from pursuing the typification of this species at this time, since we believe that it should be done in association with a comprehensive revision of species concepts established for planktonic material whilst considering all allied benthic lineages.
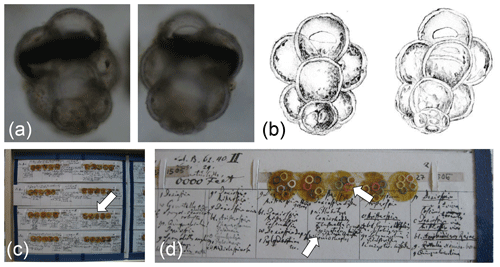
Figure 4Lectotype of Globigerinita uvula (Ehrenberg, 1862), representing the specimen figured by Ehrenberg (1873) and labelled in his handwriting as Pylodexia uvula. Light microscope image of the lectotype specimen (a) imaged from both sides of the mica mount and compared with (b) the drawing in Ehrenberg (1873). The specimen, about 0.1 mm in length, is located in the Ehrenberg Collection at the Museum für Naturkunde in Berlin on Tray 27-15B (c) on mica set 27 1506 (d), with the lectotype located on mica 3 within the red ring (white arrow) and labelled below (white arrow).
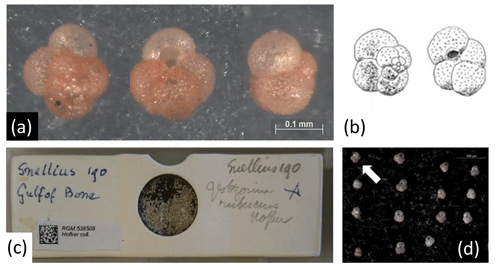
Figure 5Lectotype (a) of Globoturborotalita rubescens (Hofker 1956) compared with original drawing (b) by Hofker (1956, his plate 35, figs. 18–19), selected from a slide marked originally by Hofker as containing “Globigerina rubescens Hofker” and deposited at the Zoological Museum in Amsterdam, transferred in 2011 to the Naturalis Biodiversity Center, Leiden, the Netherlands, and registered as RGM 538508. The slide (c) contains a sieved residue from a sediment collected during the Snellius expedition (Snellius station 190) from the Gulf of Boni off Sulawesi, Indian Ocean, at 4∘43′ S, 120∘22′ E, 1407 m water depth, taken on 15 February 1930 18.15–20.30 with an Ekman–Pratje sampler. The residue hosted 15 specimens of G. rubescens that were all extracted (d), of which we here designate RGM 1333481 as the lectotype (a; white arrow in d), and specimens RGM 1333482–1333495 as paralectotypes.
3.5 Other foraminifera in the plankton
Next to the holoplanktonic taxa considered in this review, other species of foraminifera are found in the plankton. Most of these represent specimens passively entrained into the water column from the benthos during discrete events like storms, or attached to seaweed and other drifting substrates. Some species of benthic foraminifera ascend into the plankton during the final stage of their life, such as Tretomphalus and Cymbaloporetta, while others may do so during early ontogeny. However, there are two small forms with serially arranged chambers consistently found in the living plankton to such a degree that they have been commonly considered fully planktonic (De Klasz et al., 1989; Kroon and Nederbragt, 1990). One of these has been classified as Streptochilus, but based on molecular genetics, Darling et al. (2009) and Kucera et al. (2017) have shown that specimens from the living plankton bearing the Streptochilus morphology are conspecific with the benthic Bolivina variabilis (Williamson 1858). Since Streptochilus has been typified by Brönniman and Resig (1971) using another Quaternary species, whose relationship to the extant taxa is not clear, it remains open whether the genus Streptochilus should be rejected as a junior synonym of Bolivina or retained as a suitable name for fossil taxa. The work by Kucera et al. (2017) indicates the existence of two distinct morphological types among the pelagic Bolivina, but their relationship to the two commonly recognized living species of Streptochilus (S. globulosus and S. globigerum) remains unclear and the entire group requires revision explicitly considering all relevant modern Bolivina species.
Similar to Streptochilus, Ujiié et al. (2008) have shown that the presumably planktonic triserial form “Gallitellia” vivans (Cushman, 1934) is genetically closely related (congeneric) with the benthic genera Stainforthia or Virgulinella, indicating that this form could also represent a benthic species with the ability to remain active in the plankton for at least the early part of its life cycle. Since the name Gallitellia Loeblich and Tappan, 1986, is pre-occupied by the coral Gallitellia Cuif, 1977, and thus a junior homonym, Özdikmen (2009) proposed Neogallitellia as a replacement. Unlike Streptochilus, we cannot yet reject the possibility that “Gallitellia” vivans forms a distinct lineage justifying generic classification. Therefore we recommend provisionally retaining a separate generic name Neogallitellia for this lineage until further (genetic) work resolves the generic placement of N. vivans among known benthic genera.
3.6 Species concepts integrating genetic and morphological data
In order to make the taxonomy of living planktonic foraminifera consistent with and applicable to sedimentary material, we refrain from a formal treatment of genetically circumscribed lineages (cryptic species). In our view these should not enter the formal zoological nomenclature, unless accompanied with demonstrable differences in shell morphology or other visible traits, such that the resulting species could be traced in the fossil record at least to some extent. This is consistent with the species concept of Barraclough (2019), who argues that genetic distinction alone should not justify the distinction of new species and considers a species to be “an independently evolving group of organisms that is genetically and phenotypically distinct from other groups”. It is important to note that “phenotype” refers to any realized traits, and theoretically, for the extant species, one could also make use of traits that are not manifested on the shell, such as differences in cellular structure, physiology, or habitat and seasonality (e.g. Faber et al., 1988). At present we refrain from using such characters, but note that in some instances, these differences could be resolved in the fossil record by elemental and isotopic signatures in the shell (e.g. Brummer et al., 2020). For a comprehensive discussion on a possible method to establish for genetically circumscribed taxa a stable nomenclature that exists outside of the Code, the reader is referred to the work by Morard et al. (2016).
Another situation arises when genetic distinction is found “post hoc” to be associated with morphological distinction (pseudocryptic species). In this case, we recommend that when a genetic type is being associated with a species name, it should be demonstrated that the observed differences are applicable throughout the distributional range of the classified species and do not represent ecophenotypic variation. Finally, we recommend abstaining from the establishment of new species names, without a thorough revision of the concepts of existing (but abandoned) species names. Indeed, previous taxonomic revisions based on genetic evidence could make use of existing species concepts, in some cases confirming rarely used taxonomic subdivisions (Weiner et al., 2015) or resuscitating species concepts that were abandoned in the past (Aurahs et al., 2011).
A special case arises when the otherwise morphologically “cryptic” species may be separated but only based on characters that can be observed on living (or recent) material. So far, traits that can only be observed among living taxa, such as cytoplasm characteristics or symbiont type (Faber et al., 1988), have never been used to differentiate species. In this way, the classification of extant planktonic foraminifera is kept congruent with that of fossil material. However, this is at the cost of the numerous genetically distinct types with distinct ecologies and biogeographies remaining taxonomically nameless (Morard et al., 2016). In some of these cases, the situation in our opinion could be treated by using subspecies to designate the distinct taxa in the recent material. As a formal species-level taxon, a subspecies requires the designation of a physical type specimen assuring it agrees with the nominate morphological species. This would have the advantage of the fossil specimens retaining the same species name as the extant ones. Such use of a subspecies would signal that the studied material is suitable to distinguish and identify them and that the subspecies which are not listed were not found. Conversely, the subspecies name left out would signal a situation where the character cannot be determined. Indeed, the first such case arose in the classification of G. ruber, where genetic evidence supports the separation of the “pink-pigmented” type, long recognized as an ecologically distinct taxon but referred to by various informal names. The case was initially left without taxonomic resolution because the key character disappears with age in fossil material (Aurahs et al., 2011), but Morard et al. (2019a) argued for the need to name the two genetically distinct lineages, identifiable in Quaternary material by shell pigmentation, and erected G. ruber albus for the type lacking pigmentation.
3.7 Recommendations for applications in operational taxonomy
With the increasing role of data syntheses in science, including in micropaleontology, it is becoming obvious that a major barrier to the replicability and interoperability (as key aspects of the FAIR principles) of census data is the lack of standardization of vocabularies (e.g. Jonkers et al., 2020), which then translates into the problem of inconsistent taxonomies. This not only refers to the trivial case of authors using idiosyncratic names or categories with an unclear relationship to named taxa. An example of a common practice that is not conducive to interoperability arises when authors present incomplete lists of species. When presented by such an incomplete list of taxa that were encountered and/or enumerated in a given sample, it is often not immediately obvious why a certain taxon is missing. This could be either because it was not found, or it was found but synonymized by the authors with another taxon, or it was not considered by the authors and the specimens belonging to a particular taxon were assigned to various taxa or left unidentified. We believe that the value of the present taxonomic scheme (Supplement 1), as well as the annotated opinion on the nature of various synonyms and other names, is that it could contribute towards more replicable and interoperable taxonomic lists and census counts. To this end, we propose that operational taxonomy proceeds following these recommendations:
-
The authors should explicitly state and cite the work(s) on which their taxonomy is based and provide a full list of species and categories that they considered (even if no specimens belonging to some of the considered taxa were found), indicating any deviations from the cited works and spelling out the full genus and species names in the tables presenting the counts or occurrences. This is essential to make the data machine-readable and to unambiguously interpret the absence of certain species in the data. To aid authors in this respect, we provide as an electronic appendix a list of all species recognized as extant in this work (Supplement 2).
Example: “This work is based on the taxonomy in Schiebel and Hemleben (2017), recognizing all of their 47 species except for T. compressa, which is here considered a synonym of T. fleisheri.”
Example: “We considered all 50 species that are recognized as extant by Brummer and Kucera (2022), reporting abundances of all, including the seven species that were not encountered in any of the analysed samples.”
-
If the authors did not or could not differentiate any particular taxon, they should provide explicit reference to this in the paper and in the dataset they generated.
Example: “In the census counts, we did not differentiate G. elongatus, which is counted together with G. ruber albus.”
-
We strongly recommend always including a category for unidentified specimens. Such a category is required for specimens lacking distinguishing features, such as damaged or abnormal specimens or for pre-adult specimens from the plankton. Where it was not possible to identify at the species level, but an assignment to a higher-level category is possible, the authors are encouraged to record such specimens separately and apply open nomenclature, stating which taxa are being subsumed.
Example: “Specimens that could not be identified because of damage or abnormalities are reported in the category unidentified.”
Example: “Individuals smaller than 0.1 mm, which appear spinose but lack further distinguishing features are here reported as unidentified spinose juveniles. This category potentially includes representatives of all the encountered spinose species.”
Example: “Small specimens of spinose foraminifera with extraumbilical aperture and a tendency towards planispiral coiling could not be consistently differentiated at the species level and are reported here in the category Globigerinella spp., likely subsuming all four extant species of the genus.”
-
Authors who decide to separate taxa or count separately certain morphological variants (such as pre-adult specimens, coiling varieties, or any of the known morphological aberrations and forms with peculiar terminal features) should indicate how these categories map onto the parent taxonomy and next to the separate counts also provide the total for the parent category.
Example: “Specimens of T. sacculifer with the distinctive sac-like final chamber are reported in a category labelled T. sacculifer with sac, remaining specimens of the species are reported as T. sacculifer without sac.”
Example: “Pre-adult specimens of P. obliquiloculata without a cortex are here reported separately as P. obliquiloculata uncrusted.”
Example: “We consistently separated small specimens with deep umbilicus within what is commonly considered as G. scitula and report these here as G. bermudezi.”
Example: “Dextral and sinistral specimens of G. truncatulinoides have been counted separately.”
-
We recommend always reporting the actual number of counted specimens rather than percentages or concentrations, alongside the total number of specimens counted, and provide information on the portion (split) of the sample that was counted and the size fraction (sieve or mesh size) that was used.
Example: “All specimens of planktonic foraminifera were counted in the fraction > 0.1 mm. Where the total number of specimens in a sample exceeded 500, the sample was split and the portion analysed is recorded alongside the actual counts in Table 3 and in the electronic supplement.”
-
Authors are asked to provide the information on all of the above and on all metadata associated with the counted assemblage and the material from which it derives together with the counts in a digital repository, so future users can interpret the taxonomy and the counts without searching for this information in the literature in which it may or may not be mentioned.
-
At any time when the taxonomy is revised, the authors are asked to avoid changes, as far as possible, which result in a name being assigned a new meaning which conflicts with previous usage.
Despite half a century of their extensive applications in the field of paleoceanography, the taxonomy of extant and late Quaternary planktonic foraminifera is not yet entirely stable. Some of the recent amendments reflect only the necessity to change a name or transfer a species, without questioning the underlying morphospecies concepts (such as the establishment of Trilobatus by Spezzaferri et al., 2015), but others attest to the incomplete understanding of the nature of morphological variability within species (such as the reinstatement of Globigerinoides elongatus by Aurahs et al., 2011). Both aspects combined contribute to the ongoing lack of consistency in taxonomic identifications (e.g. Al-Sabouni et al., 2018; Fenton et al., 2018), hampering biodiversity and paleoecology studies as well as efforts to generate community resources to automate species identification to achieve high throughput (Hsiang et al., 2019; Mitra et al., 2019). We hope the taxonomic benchmark provided in this study will help contribute to the development of a more effective and robust taxonomy of extant, and indirectly also fossil, species of this important group of marine microfossils.
We realize that this contribution is not the last word on the taxonomy of modern planktonic foraminifera. Indeed, in many instances, we anticipate revisions resulting from new molecular evidence as well as from ontogenetic morphology (by 3D microtomography) or revision of fossil taxa and types, and we highlight these instances explicitly in the annotations. In parallel with the classical approach of taxonomic revisions being presented in formal publications, the last decade has seen the rise of internet-based resources, allowing simultaneous access to species descriptions, taxonomic opinions, images and stratigraphic data. Prime examples for planktonic foraminifera are the Mikrotax system for web delivery of taxonomy (Huber et al., 2016; Young et al., 2020) and the World foraminifera database (Hayward et al., 2020) maintained as a part of the World Register of Marine Species (WoRMS Editorial Board, 2020), linked with the GBIF biodiversity information facility (GBIF.org, 2020). These resources are complemented by providers of specific information such as the images database Foraminifera.eu (Hesemann, 2015) or the PFR2 database of SSU rDNA barcode sequences of extant planktonic foraminifera (Morard et al., 2015). Whilst there will always be space for classical taxonomic revisions, especially for the purpose of formal documentation of taxonomic acts, internet-based taxonomic resources will likely become the main reference for everyday taxonomic work, including for biostratigraphical species identification or paleoecological census counts. We highly endorse these efforts and call the community to contribute by sharing images and data and by citing these resources whenever appropriate.
In the following, we provide a complete annotated list of all 24 genera and 50 species and subspecies of modern planktonic foraminifera, which we consider to be found among the living plankton. We highlight important taxonomic observations for selected taxa and discuss key synonyms and other names with reference to the taxonomies presented previously (e.g. Schiebel and Hemleben, 2017; Hemleben et al., 1989; Saito et al., 1981) when of added value. Genera are sorted alphabetically as are the species within them. The taxonomic relationships among the genera are shown in Fig. 2.
-
Beella Banner and Blow, 1960
Initially considered a subgenus of Globorotalia by Banner and Blow (1960b), because of its distinct apertural shape, but a relationship to Globigerina was firmly established by Parker (1962). Kennett and Srinivasan (1983) also derived the genus from Globigerina, but genetic evidence indicates that it diverged from Globigerinella (Weiner et al., 2015). The genus is retained here because it has well resolved fossil history and allows clear demarcation of its type species Beella digitata (Brady, 1879) from the homonymous Hastigerinella digitata (Rhumbler, 1911). One extant and several fossil species.
-
Beella digitata (Brady, 1879)
A rare but distinct extant species typified by a specimen from recent sediments. As shown already by Parker (1962), the characteristic chamber elongation is less well developed in small specimens. This has led some authors (e.g. Aze et al., 2011) to separate these forms as Beella megastoma (Earland, 1934) or consider the extinct ancestral Beella praedigitata (Parker, 1967) to persist to the present. The existence of the homonymous Hastigerinella digitata (Rhumbler, 1911) has created some confusion in the past, with both species being occasionally assigned to the same genera (Globigerinella, Hastigerina).
-
Berggrenia Parker, 1976
Typified by the extinct Berggrenia praepumilio (Parker, 1967), the phylogenetic position of this genus remains enigmatic. It features an adumbilically displaced ampullate final chamber, like the spinose Turborotalita, but its wall is non-spinose with the umbilical side ornamented by radial striae and pores concentrated along the sutures on the spiral side. For example, Saito et al. (1981) emphasized the ampullate final chamber to assign to Berggrenia the minute Turborotalita clarkei, a decision which we here reject. We consider it likely that Berggrenia represents a lineage of foraminifera that invaded the plankton from an independent benthic ancestor, and the genus must be retained until its phylogenetic position is resolved by genetic data. One extant and one fossil species.
-
Berggrenia pumilio (Parker, 1962)
Small and rarely recorded species with distinct morphology and ornament, initially assigned by Parker (1962) to Globorotalia. This species has always been elusive, but it has been reported from the plankton (e.g. Rebotim et al., 2017) and when pictured always features the typical morphology and ornament (Saito et al., 1981; Schiebel and Hemleben, 2017).
-
Bolivina d'Orbigny, 1839
This genus comprises species with biserially arranged chambers, most of which are considered benthic, but one extant lineage appears to also live in the plankton during part of its life cycle (Darling et al., 2009). To what degree this also applies to fossil species of Bolivina and whether or not the abundant Neogene fossil biserial planktonic foraminifera should also be assigned to this genus remains unclear. One extant species in the plankton, many extant and fossil benthic species.
-
Bolivina variabilis (Williamson, 1858)
Abundant and ubiquitous species occurring in outer shelf and upper slope sediments and in the plankton of tropical to temperate oceans (Darling et al., 2009; Kucera et al., 2017). No type appears to have been designated, but the species was described from recent sediments off the British Isles, indicating that the name should be associated with an extant species, and the species concept has remained stable ever since. Designation of a type would require a thorough revision of species concepts invoked for material derived from the plankton, combined with the evaluation of other morphologically similar species described from the benthos.
-
Candeina d'Orbigny, 1839
One of the three still valid genera erected by d'Orbigny (1839a). Typified by the extant Candeina nitida d'Orbigny, 1839, the genus is distinct and it has been always easy to distinguish it, but it proved difficult to fit it into the phylogenetic system (see statements to this end in Parker, 1962, and Saito et al., 1981). Parker (1962) considered earlier classification attempts “illogical” and following Hofker (1954) suggested a link with Globigerinita. This classification of Candeina is indeed consistent with its microperforate wall and early ontogeny (Brummer, 1988a), as well as with genetic evidence indicating that this genus is related to Globigerinita (Ujiié and Lipps, 2009; Morard et al., 2019b). Following most recent works but unlike Pearson et al. (2018), we here retain Candeina among the microperforate clade. One extant and several extinct species.
-
Candeina nitida d'Orbigny, 1839
One of the three extant species lacking a type, with the original material considered lost. Morphologically distinct and its species concept undisputed, we feel it is not essential to designate a neotype at this stage.
-
Dentigloborotalia Brummer, 1988
Characterized by compressed reniform chambers and wall texture with shark-tooth like pustules, this monotypic genus has been established to accommodate the peculiar species D. anfracta. Supported by molecular genetic data (Morard et al., 2019b), we consider it likely that Dentigloborotalia represents a lineage of foraminifera that invaded the plankton from an independent benthic ancestor and the genus must be retained until the phylogenetic position of the species relative to its benthic ancestors is resolved.
-
Dentigloborotalia anfracta (Parker, 1967)
A distinct species found abundantly in the plankton and in sediments, but recorded rarely because of its small size (Brummer, 1988b). When recognized, the species concept is mostly applied consistently (Saito et al., 1981). Holotype from recent sediments represents the typical morphology with reniform chambers.
-
Globigerina d'Orbigny, 1826
The most iconic and the earliest described genus of planktonic foraminifera, typified by the well-established Globigerina bulloides d'Orbigny, 1826. Originally used widely for almost all species considered planktonic, now limited to a lineage of spinose planktonic foraminifera with a finely spinose wall and single umbilical aperture (Spezzaferri et al., 2018a). Two extant and a number of fossil species.
-
Globigerina bulloides d'Orbigny, 1826
The concept of the species was initially applied more broadly but with the description of new species it progressively converged towards the current understanding of the taxon and remained stable since. d'Orbigny erected G. bulloides and G. elongata formally as replacement names for species illustrated by Soldani (1791) in a non-Linnean and thus unavailable work. Although d'Orbigny provided no description, he validated the species by referring to Soldani's (1791) illustrations. Guided by plaster models made by d'Orbigny, G. bulloides was typified by a lectotype selected by Banner and Blow (1960a). The species is abundant in productive waters along the Equator, in temperate regions and in all upwelling regions, often showing strong seasonality. It harbours substantial genetic diversity (Darling et al., 2017), and it is possible that some of the constituent genetic types will prove pseudocryptic, vindicating the use of some of the numerous names considered here as morphological variants within the species (such as G. umbilicata or G. cariacoensis).
-
Globigerina falconensis Blow, 1959
Although the species resembles G. bulloides, causing frequent confusion (Al-Sabouni et al., 2018), the two species are distinct morphologically (Malmgren and Kennett, 1977) and ecologically, and when applied to modern plankton, the concept of G. falconensis is used consistently. Unfortunately, the species is typified by a specimen from Miocene deposits in Venezuela. The overall morphology of the holotype is consistent with the present concept of G. falconensis, but a recent investigation revealed consistent morphological differences between the Miocene and Recent populations of the lineage (Bridget Wade and Alessio Fabbrini, personal communication, 2021), indicating that the species name may require revision.
-
Globigerinella Cushman, 1927
Synonymized with Hastigerina by Bolli et al. (1957) and Banner and Blow (1960b), the two genera were separated again because of differences in wall texture (Saito et al., 1981; Kennett and Srinivasan, 1983). In its present concept, the genus refers to a lineage descending from Globigerina with extraumbilical to equatorial apertures and a tendency towards planispiral coiling (Spezzaferri et al., 2018a). Typified by the extant Globigerinella siphonifera (d'Orbigny, 1839), the genus is paraphyletic, giving rise to several genera characterized by the development of radially elongated chambers, including the here accepted Beella, the here rejected Bolliella and the fossil Protentella, rejected by Spezzaferri et al. (2018a). Four extant and many fossil species.
-
Globigerinella adamsi (Banner and Blow, 1959)
Classified as Bolliella adamsi by Schiebel and Hemleben (2017), but the rarely used, strictly monotypic and phylogenetically unnecessary genus Bolliella is here rejected. A rare element of Indo-Pacific tropical plankton, possessing distinct adult morphology, but pre-adult specimens may be difficult to distinguish from other species of Globigerinella.
-
Globigerinella calida (Parker, 1962)
Erected by Parker (1962) to differentiate smaller, less planispiral forms with radially elongated chambers from the more planispiral and larger Globigerinella siphonifera. The two species have a distinct ecology, and the G. calida morphology is associated with a specific genetic lineage within the genetically diverse Globigerinella (Weiner et al., 2015), justifying the retention of this species. However, the morphological separation from G. siphonifera is often gradual and the identification is confounded by the existence of Globigerinella radians, making it difficult to apply the species concept consistently (Al-Sabouni et al., 2018).
-
Globigerinella radians (Egger, 1893)
Resurrected by Weiner et al. (2015) to resolve a taxonomic confusion resulting from the apparently parallel evolution of radially elongated chambers in this species and G. calida (and for that matter in G. adamsi). Banner and Blow (1960b) were the first to indicate affinity of this obscure Ehrenberg's species to the Globigerinella clade by assigning it to their newly erected subgenus Beella. No type has been designated and Egger's material from the German Gazelle expedition, initially deposited at the Bayerische Staatssammlung in München, appears to have been destroyed during the Second World War and is thus considered lost. It is possible that further genetic work on Globigerinella will provide new evidence requiring further revisions within the genus.
-
Globigerinella siphonifera (d'Orbigny, 1839)
Frequently referred to as Globigerinella aequilateralis (Brady, 1879). The synonymy of the two species has been established already by Banner and Blow (1960a), who also selected suitable lectotypes based on original material of d'Orbigny and Brady. Their decision to exhume the name “siphonifera” was grudgingly accepted by Parker (1962), who resented that d'Orbigny's name had been disinterred by Banner and Blow (1960a) but conceded the need to now reject the popular G. aequilateralis. Unfortunately, other authors such as Saito et al. (1981, p. 28) maintained that G. siphonifera was a nomen oblitum (which was clearly incorrect at that time, considering the usage by Parker, 1962) and retained G. aequilateralis, and this decision was followed by key works such as by Kennett and Srinivasan (1983), explaining the long history of parallel names. The apparent phylogenetic placement of G. calida, the establishment of G. radians, and likely the existence of G. adamsi all appear to make this genetically hyperdiverse species (Weiner et al., 2014) in its present concept paraphyletic (Weiner et al., 2015).
-
Globigerinita Brönnimann, 1951
Typified by Globigerinita naparimaensis Brönnimann, 1951, which was later accepted as a synonym of the extant Globigerinita glutinata (Egger, 1893). For a discussion on the synonymy, the reader is referred to the study by Parker (1962). This microperforate genus was initially applied to various species possessing a bulla, irrespective of wall texture (Bolli et al., 1957), but was refocused on the microperforate species by Parker (1962), although she at that time also included in Globigerinita the macroperforate and spinose T. humilis. In its current concept, the genus refers to Oligocene to extant microperforate taxa with an umbilical aperture (Pearson et al., 2018). The genus is paraphyletic, giving rise to Candeina. Three extant species.
-
Globigerinita glutinata (Egger, 1893)
The original description of the species by Egger (1893) is sufficiently detailed to confirm that the name refers to a microperforate species (Egger specifically highlighted the felt-like surface of the shell) with a morphology consistent with its modern representatives. A type has never been designated and our own enquiries at the Bayerische Staatssammlung in München failed to locate Egger's material, derived from the German Gazelle expedition, which must therefore be considered lost. Since the concept of the species is not contested and has been used consistently on modern material, we leave the designation of a type for future work. The considerable morphological variability in the species, especially in terms of the different development of the bulla, has led to a proliferation of names. Following the practice initiated by Parker (1962) and followed by Pearson et al. (2018), these forms are here all considered as synonyms. However, we note that the species is genetically diverse (Morard et al., 2019b), and it is at present not clear if the genetic types are associated with morphological differences.
-
Globigerinita minuta (Natland, 1938)
Like in many other species referable to Globigerinita, the species was originally misinterpreted with the multiple infralaminar apertures leading Natland (1938) to assign his new species to Globigerinoides. An SEM rendering of the holotype published by the National Museum of Natural History (Smithsonian, Washington DC, United States) confirms that it is microperforate and less trochospiral than in the illustration by Natland (1938). This is consistent with the observations by Parker (1962), who synonymized G. minuta with G. uvula but noted the lower spire in the holotype of G. minuta, referring to specimens on her plate 8, figs. 24–26, which we indeed consider consistent with G. minuta. Despite the overall similarity with G. uvula, the species is genetically distinct and this distinction appears to be associated with moderately high-spired specimens bearing a characteristic highly arched aperture and showing a different ontogenetic trajectory (Brummer, 1988a; Morard et al., 2019b), consistent with the concept by Saito et al. (1981) and Schiebel and Hemleben (2017).
-
Globigerinita uvula (Ehrenberg, 1862)
The specimen of G. uvula, which was illustrated by Ehrenberg (1873), is preserved at the Museum für Naturkunde in Berlin. The sheet with the original drawing contains information that facilitated the identification of the drawn specimen in one of Ehrenberg's mica mounts (Ehrenberg mounted samples of deep-sea sediment akin to smear slides in Canada Balsam on thin mica sheets and indicated the position of pictured specimens by small coloured paper rings stuck on the mount). Following recommendations by the Code, this specimen (Fig. 4) can be considered syntypic and is here designated as the type (lectotype), analogous to the designation of the lectotype of N. pachyderma by Darling et al. (2006). The species has been often referred to as Globigerinita bradyi (Wiesner, 1931), which is a junior synonym (Parker, 1962; Saito et al., 1981). Like for N. pachyderma, the year of description has been often erroneously stated as 1861.
-
Globigerinoides Cushman, 1927
Typified by the extant Globigerinoides ruber (d'Orbigny, 1839), the genus refers to a diverse and abundant warm-water clade of spinose species with supplementary apertures (Morard et al., 2019a). To assure the monophyly of Globigerinoides, Spezafferri et al. (2015) transferred the extant sacculifer-plexus to their newly erected genus Trilobatus. The genus name Globigerinoides is grammatically masculine, and after quite some discussion decades ago, the grammatical gender of the constituent species has been adjusted accordingly and stabilized (e.g. Globigerinoides rubra becoming Globigerinoides ruber). However, the fourth edition of the Code contains an amendment to article 30, which deals with gender-group words of this type, in a way that could be interpreted as implying that the name should be feminine. This is because it opens up the possibility for names created with the ending -oides, which are grammatically masculine, to be considered of different gender, if the original author stated so, for example by combining it with a species name in another gender form. In the interest of nomenclatural stability, we refrain from applying this amendment to Globigerinoides. Five extant and many fossil species and subspecies.
-
Globigerinoides conglobatus (Brady, 1879)
A well-established species, with adequate type from recent sediments. Morphologically variable, with large changes in chamber shape through ontogeny, but appears genetically homogenous in the modern ocean (Morard et al., 2019a).
-
Globigerinoides elongatus (d'Orbigny, 1826)
Synonymized with Globigerinoides ruber by Parker (1962), causing a proliferation of informal names for morphotypes within the resulting broad concept of G. ruber, including the popular G. ruber sensu lato of Wang (2000). Reinstated in a combined morphological and genetic investigation by Aurahs et al. (2011), the species is distinguished by an asymmetrically flattened (compressed) final chamber and occasionally also the penultimate chamber. Type specimen with representative morphology was designated by Banner and Blow (1960a) from a region where the genetic type associated with this name and morphology has been found to occur (Aurahs et al., 2011). Numerous synonyms, or partial synonyms, highlighting different degrees of spire elongation (a character also occurring in G. ruber), chamber compression and aperture size. Specimens figured by Schiebel and Hemleben (2017) on plate 2.8, figs. 12–16 as G. ruber have to be considered G. elongatus.
-
Globigerinoides ruber albus Morard et al., 2019
Established to differentiate the extant lineage of G. ruber without the red pigmentation from the ecologically and genetically distinct lineage with pigmentation (Morard et al., 2019a). The only case in planktonic foraminifera where the type specimen (shell) is associated with a DNA barcode (a fragment of the SSU rDNA extracted from the cytoplasm within the shell). Possessing the typical G. ruber morphology with inflated chambers, G. ruber albus is consistent with Globigerinoides ruber sensu strictu of Wang (2000). The large morphological variability, long-term confusion with Globigerinoides elongatus (see Aurahs et al., 2011) and existence of aberrant morphologies has lead to the establishment of many synonyms. None of these appear justified at present, but we note that G. ruber albus harbours several apparently cryptic genetic types (Morard et al., 2019a), and a further revision may be justified if some of these prove pseudocryptic.
-
Globigerinoides ruber ruber (d'Orbigny, 1839)
Referring to the conspicuous reddish pigmentation of the shell, d'Orbigny's name must be the nominotype for the subspecies designating the genetic lineage of G. ruber where the pigmentation occurs (Morard et al., 2019a). Indeed, Banner and Blow (1960a) designated and figured a lectotype of G. ruber, noting that “the earlier part of the test is red coloured”. The name is thus safely associated at the subspecies level with the red-pigmented form of the otherwise genetically and morphologically diverse species.
-
Globigerinoides tenellus Parker, 1958
An unusually small species of the genus Globigerinoides, despite possessing a supplementary aperture on the final chamber for decades considered to belong to Globoturborotalita. Identified genetically as a sister species to G. elongatus and thus transferred to Globigerinoides by Morard et al. (2019a).
-
Globoquadrina Finlay, 1947
Typified by the extinct species Globoquadrina dehiscens (Chapman et al., 1934), the assignment of the only modern representative, G. conglomerata, to this genus is problematic. Wade et al. (2018b) transferred the species to Dentoglobigerina, leaving the previously species-rich Globoquadrina monotypic. The extant species, endemic to the tropical Indo-Pacific, is genetically distinct from all other non-spinose taxa (Morard et al., 2019b), requiring classification in a distinct genus. Because Dentoglobigerina is spinose according to Wade et al. (2018b), we cannot assign the extant and ostensibly non-spinose species to this genus. Therefore, we here provisionally retain Globoquadrina, awaiting revision of the fossil species leading to the modern taxon. As applied here the genus contains one extant and at least one fossil species.
-
Globoquadrina conglomerata (Schwager, 1866)
A conspicuous modern species, which is commonly thought to have descended from the Neogene Globoquadrina venezuelana (Kennet and Srinivasan, 1983; Aze et al., 2011). Wade et al. (2018b) reexamined the types of G. conglomerata and found them to be conspecific with G. venezuelana, which is consistent with observations by Banner and Blow (1960a) and Parker (1962) that the two species are similar and potentially synonymous. It remains to be established whether the topotypic material, presumably of Pliocene or upper Miocene age, including the neotype selected by Banner and Blow (1960a), is consistent with the modern species. If it is not, then the modern species needs a new name. If it is consistent, as it seems from the illustrations, then the name G. conglomerata would have priority over G. venezuelana. Either way, the taxonomic conundrum spills over to the generic assignment of the species, as Wade et al. (2018b) transferred G. venezuelana together with G. conglomerata to the genus Dentoglobigerina, which they consider spinose. Since there is no evidence for spines in living G. conglomerata, and molecular genetic data do not show any evidence for a relationship with the spinose clade (Morard et al., 2019b), we here choose to retain this modern non-spinose species in Globoquadrina but anticipate a need for revision of modern and fossil representatives of this lineage.
-
Globorotalia Cushman, 1927
Typified by the extant Globorotalia tumida (Brady, 1877), the genus was initially used for a range of extant and fossil taxa with compressed shells and/or an extraumbilical aperture. Once it became obvious that these features evolved independently several times, the genus concept was gradually narrowed to a single Neogene lineage leading to the nominotype species. The concept was still too broad at the time of the revision by Parker (1962) but assumed a similar form to modern usage by the revision in Kennett and Srinivasan (1983), albeit still including their Jenkinsella, which is now known to be unrelated (Leckie et al., 2018). With 10 extant and many fossil species, the genus is unusually diverse. It comprises several distinct lineages, which have been treated as subgenera, and the subgenus names were later sometimes raised to genera. We here retain a single genus, because the use of other generic names has little tradition among extant planktonic foraminifera and the subdivision of the genus is not necessary, as the genus in the broad concept remains monophyletic. We note that many of the erected subgenera may be useful, especially when tracing the phylogeny of the extant species (e.g. Kennett and Srinivasan, 1983; Aze et al., 2011). However, we point out that the subgenera are riddled with synonyms and homonyms and their reinstatement would require a careful revision of the nomenclature (Table 1).
-
Globorotalia cavernula Bé, 1967
Since its original description by Bé (1967b), G. cavernula has been elusive. Nevertheless, this species is retained because of the extensive documentation of its distinct morphology and relatively high abundance in the plankton claimed by the original author. The holotype indicates that the species is related to G. truncatulinoides, and the lack of occurrence records elsewhere indicates that it may be restricted to cold waters of the South Pacific. It is possible that G. cavernula refers to one of the known cryptic species of G. truncatulinoides (Quillévéré et al., 2013).
-
Globorotalia crassaformis (Galloway and Wissler, 1927)
This species is typified by a specimen derived from Pleistocene sediments, from well within its commonly accepted stratigraphic range (Kennett and Srinivasan, 1983) and showing morphology entirely consistent with its living representatives. An important element of sub-thermocline faunas in tropical to temperate regions, the species is morphologically diverse and a host of names exists for various forms (see Saito et al., 1981). These are all here considered synonyms with respect to the extant G. crassaformis, but we note that for example Aze et al. (2011) considered Globorotalia oceanica Cushman and Bermúdez, 1949, as extant and lists five additional species allied to G. crassaformis as extinct. Genetic data are so far too scarce to decide whether pseudocryptic diversity exists within the species.
-
Globorotalia hirsuta (d'Orbigny, 1839)
A neotype was designated by Blow (1969) from recent sediments, and a second (later invalidated) neotype has been designated by Le Calvez (1974). The species is abundant in temperate waters and appears morphologically as well as genetically homogenous.
-
Globorotalia inflata (d'Orbigny, 1839)
A distinct and abundant extant species, typified by a specimen from recent sediments designated by Banner and Blow (1967). A second (later invalidated) neotype has been designated by Le Calvez (1974). Two distinct pseudocryptic species (Morard et al., 2011) indicate a potential for future revision.
-
Globorotalia cultrata (d'Orbigny, 1839)
Whereas the morphological concept of this conspicuous extant species remained relatively stable, the name has a turbulent history. The confusion arose from the inconsistent usage of d'Orbigny's (1826) name Rotalia (Rotalie) menardii, which was, like almost all other names in that work, initially a nomen nudum and thus invalid. However, a description was later provided by Parker et al. (1865), which, unfortunately, separated the name from the original concept by d'Orbigny such that it became synonymous with d'Orbigny's (1839a) G. cultrata. This created the impression that both names refer to the same entity, with G. menardii having priority by ascribing its authorship incorrectly to d'Orbigny (1826). This view has been cemented by Banner and Blow (1960a) designating a neotype for G. menardii from recent sediments, which is representative of the extant species and thus conspecific with G. cultrata. The controversy over the nomenclatural priority of G. menardii is covered in detail by Banner and Blow (1960a), Parker (1962) and Stainforth et al. (1975). Stainforth et al. (1975) decided to resolve the situation by insisting that G. menardii should be used for Miocene biconvex forms, because the material that d'Orbigny (1826) studied could have only referred to that form. The discussion resulted in a formal ICZN case and ruling (Stainforth et al., 1978: ICZN Case 2145; ICZN Opinion 1234, 1982), which re-attached the name G. menardii to the original concept of d'Orbigny, referring to an extinct Neogene Mediterranean form, and the authorship has been fixed to d'Orbigny in Parker et al. (1865). Unfortunately, this ruling has been misunderstood as a complete suppression of the name G. cultrata, and the name G. menardii continued to be used for the extant species as well, which is clearly at variance with the intention of Stainforth et al. (1975, 1978). Upon review of the relevant literature, we must conclude that the validly described and available name Rotalina cultrata d'Orbigny 1839, fixed by a highly representative type specimen derived from recent sediments (Fig. 6) and conspecific with what is commonly considered as the extant G. menardii, must be reinstated for the extant species.
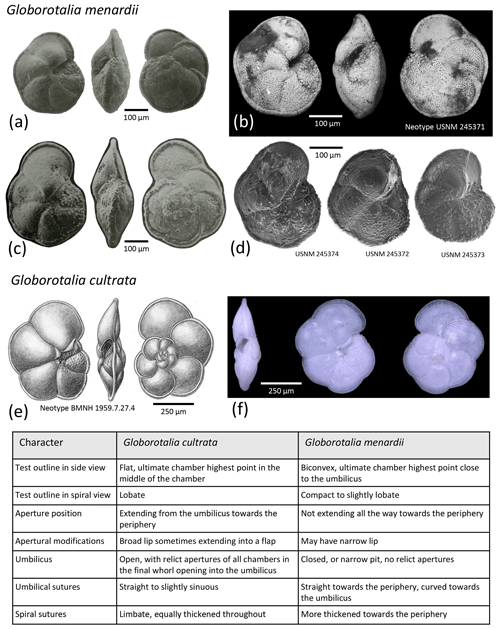
Figure 6Neotype of Globorotalia menardii (Parker, Jones and Brady, 1865) as designated here with (a) being the specimen imaged by Stainforth et al. (1978) on their plate 2, fig. 1, and (b) the specimen labelled as type and deposited as such by Stainforth in the USNM collections and here designated as neotype. The specimen initially designated by Stainforth et al. (1978) as the neotype (c) deviates from all other specimens deposited by Stainforth at the USNM (a, d) and must be considered lost. (e) Neotype of Globorotalia cultrata (d'Orbigny, 1839) as designated and drawn by Banner and Blow (1960a), compared to (f) a light microscope image of a topotypical specimen extracted from Caribbean surface sediments (image by Adrian Baumeister). The table below the illustrations is listing key morphological characters differentiating the two species.
The initial concept of d'Orbigny, where G. menardii was based on material that is now considered Neogene, made Stainforth et al. (1975) designate a Miocene neotype (Fig. 6), which was upheld by ICZN ruling (ICZN Opinion 1234, 1982) over the Quaternary lectotype selected for G. menardii by Banner and Blow (1960a). Remarkably, on examination of the type material in the Smithsonian Institution, the specimen designated by Stainforth as neotype and deposited in the collections as USNM245371 is quite obviously not the same specimen as the neotype designated by Stainforth et al. (1978; their plate 1, fig. 1) and upheld by the ICZN ruling. Instead, this specimen is identical to that which Stainforth et al. (1978) depict on their plate 2, fig. 1 (Fig. 6). Next to the specimen labelled as neotype, Stainforth deposited three further specimens at the Smithsonian Institution, which have been imaged courtesy of the curator Brian Huber and are depicted here in Fig. 6. None of these correspond to the neotype of Stainforth et al. (1978), and two show damage to the last chamber as commonly occurs when specimens are being removed from an SEM mount. There are no other specimens deposited in the USNM collections on the occasion of the type designation for G. menardii. In the opinion of the curator, which we share, the formally designated neotype was damaged during handling and replaced by Stainforth. Therefore, we conclude that the neotype designated by the ICZN Opinion 1234 (1982) ruling must be considered lost and we here designate the specimen deposited under USNM245371 as a replacement neotype. The specimen clearly originates from the same type series as the ICZN type and was labelled as type by the author. It possesses representative morphology and is undamaged.
In the concept of Stainforth et al. (1975, 1978), as also evidenced by their selection of type, G. menardii is distinct from the extant species, and in their revision, Stainforth et al. (1975) clearly state that their G. menardii refers to a Miocene form from the Mediterranean, distinct from modern representatives of the lineages, which they considered as G. cultrata. The Miocene form is commonly found in Neogene Mediterranean sediments, with a well recorded and stratigraphically useful last occurrence in the late Miocene throughout the Mediterranean (Lirer et al., 2019). Unfortunately, the confusion between G. menardii and G. cultrata has not been resolved as intended by the ICZN ruling, and both names continued to be in use for extant material, either as synonyms or as names for morphological variants (e.g. Regenberg et al., 2010), with G. menardii being the more common designation, adopted by authoritative works (Kennett and Srinivasan, 1983; Hemleben et al., 1989) and perpetrated by almost all users, including us. With G. menardii in the concept of Stainforth et al. (1975, 1978) being placed on the Official List of Species Names by the ICZN (Name Number 2832), the name cannot be applied to extant taxa without a new ruling by the ICZN, which would have to overrule its own earlier decision. This causes a situation where the stability of the nomenclature requires a change, which is in conflict with prevailing usage. Unfortunately, we feel compelled to implement this change, and we here urge the community to adopt the name G. cultrata for the distinct and abundant extant form and abandon the erroneous usage of G. menardii, which clearly refers to an extinct form, with a distinct morphology (Fig. 6).
With respect to the proposed change in usage, we believe that it is safe to assume that all references to G. cultrata to date refer to our G. cultrata, as do all references to G. menardii applied on extant or Quaternary material. References to G. menardii in material older than Quaternary may refer either to our G. cultrata or to the extinct G. menardii of Stainforth et al. (1975, 1978). Although we have not ventured to clarify the phylogenetic relationship of the two taxa in the fossil record, we note a number of distinct morphological features allowing in our opinion unambiguous diagnosis of the extant G. cultrata against the Mediterranean Miocene G. menardii (Fig. 6). The extant Globorotalia cultrata has been confirmed by molecular genetic data to be distinct from other globorotaliids, with no evidence for genetic diversity among its extant populations (Morard et al., 2015) to support the commonly used subspecies such as G. menardii flexuosa (the type of this subspecies indicates that it originally referred to G. tumida) and G. menardii fimbriata, which in our opinion both refer to extinct taxa, morphological variants or aberrations without taxonomical significance.
-
Globorotalia scitula (Brady, 1882)
Lectotype selected by Banner and Blow (1960a) based on the original surface sediment material from Brady (1982). Small forms with an open umbilicus, occurring in modern plankton, have been described as Globorotalia bermudezi Rögl and Bolli, 1973, and this species was considered as extant by Aze et al. (2011). There could well turn out to be (pseudo)cryptic species in G. scitula, in which case G. bermudezi may be reinstated, but at present there is no strong evidence for morphological discontinuity within the extant representatives of the species.
-
Globorotalia eastropacia Boltovskoy, 1974
Specimens of this distinct Indo-Pacific form have been initially assigned to other species, but many authors noted the differences to established taxa. Parker (1962) assigned this form to G. hirsuta but noted that these specimens belonged to a distinct group (Group 3, her plate 5, figs. 13 and 15) characterized by flat spiral side and poorly developed keel, occurring frequently in the Indo-Pacific. Saito et al. (1981) erroneously synonymized with this species the figured specimens of group 1 by Parker (1962, her plate 5, figs. 12 and 14), which Parker (1962) clearly considered “typical” of G. hirsuta as found around the Canary Islands. The same morphological distinction led Boltovskoy (1974) to describe Globorotalia hirsuta eastropacia from plankton tows in the Pacific. His description was published in January 1974, clearly referring to the same species described as Globorotalia theyeri by Fleisher in a work published in April 1974 (Fleisher, 1974). Hence the more commonly used name G. theyeri is a junior synonym, and in the absence of a strong case against G. eastropacia, which has been occasionally used (e.g. Saito et al., 1981), the name G. eastropacia should be adopted instead.
-
Globorotalia truncatulinoides (d'Orbigny, 1839)
Since the type specimen of Rotalina truncatulinoides from d'Orbigny (1839b) was lost, Blow (1969) designated a neotype from recent sediments. An invalid, since later, lectotype has been designated by Le Calvez (1974). The general concept of this distinct and abundant deep-dwelling species has remained stable, but the large intraspecific morphological variability (e.g. Lohmann and Malmgren, 1983) led to the proliferation of names referring to various subfossil and extant forms. Aze et al. (2011) recognize two extant and three extinct species with a morphology falling within the range of variability of extant G. truncatulinoides, whereas Kennett and Srinivasan (1983) followed a more conservative approach close to the current species concept. The species harbours at least five distinct genetic types, which appear to be associated with morphological differences (Quillévéré et al., 2013), heralding the potential for future revision.
-
Globorotalia tumida (Brady, 1877)
Type species of Globorotalia, itself typified by a lectotype selected by Banner and Blow (1960a) from presumably Quaternary or Pliocene rock fragments collected on a beach of New Ireland after a tsunami. The species has been often lumped with G. cultrata (then referred to as G. menardii) in studies based on quantitative census counts of Quaternary assemblages. This was due to a combination of the taxonomic confusion between the fossil G. menardii and modern G. cultrata (as lamented by Parker, 1962) and by ignoring at the same time also the distinct G. ungulata. The current species concept is consistent with Saito et al. (1981) and Kennett and Srinivasan (1983) and includes specimens referred to as Globorotalia flexuosa (Koch 1923), which we consider to represent a morphological aberration as long as the name is applied on extant species.
-
Globorotalia ungulata Bermúdez, 1961
Often ignored, but representing a necessary category to correctly assign specimens of the G. tumida–G. cultrata plexus. Applied consistently with current understanding by Saito et al. (1981) and Kennett and Srinivasan (1983). Specimen figured by Schiebel and Hemleben (2017) on plate 2.30 and figs. 1–3 represents G. ungulata, not G. tumida. Typified by a holotype from recent sediments and confirmed by molecular genetic data to be distinct, but related to G. tumida (Morard et al., 2015).
-
Globorotaloides Bolli, 1957
Typified by the extinct species Globorotaloides variabilis Bolli, 1957, which has been connected with some confidence to a lineage leading to the extant species (Coxall and Spezzaferri, 2018). A distinct dominantly Indo-Pacific form with two extant and many fossil species.
-
Globorotaloides hexagonus (Natland, 1938)
Distinct deep-water form found in the plankton only in the Indo-Pacific. The Quaternary holotype refers to a morphology with straight dorsal sutures, frequently observed in the sediments and consistent with the concept by Parker (1962).
-
Globorotaloides oveyi Buckley, 1973
A second form among the extant Globorotaloides, characterized by curved dorsal sutures and more compressed chambers was observed in plankton samples from the Indian Ocean by Buckley (1973), leading him to the establishment of a new species. Remarkably, Saito et al. (1981), otherwise consistent splitters, have considered G. oveyi synonymous with G. hexagonus. However, considering the distinct morphology of G. oveyi, its occurrence in the plankton and our own observations from the plankton (Fig. 7), we see no grounds on which to reject G. oveyi as a distinct species, but note that this decision requires confirmation by genetic data.
Figure 7SEM images of Globorotaloides collected during the M74 1b expedition (Bohrmann et al., 2010) in the Arabian Sea, showing shell morphology consistent with the original description of (a) Globorotaloides hexagonus (Natland) and (b) Globorotaloides oveyi (Buckley). Both specimens appeared alive (with coloured cytoplasm) when collected. Specimen in (a) is from station 947, 300–500 m water depth; that in (b) is from station 945, 500–700 m water depth.
-
Globoturborotalita Hofker, 1976
Typified by the only extant species of the genus, Globoturborotalita rubescens (Hofker, 1956), Globoturborotalita comprises a large number of fossil species, all characterized by small globular shells with inflated chambers and coarse, cancellate wall texture (Spezzaferri et al., 2018b). The extant species now classified as Globigerinoides tenellus has been assigned in the past to Globoturborotalita (e.g. Schiebel and Hemleben, 2017; Spezzaferri et al., 2015), being the only species of the genus with supplementary aperture. The species was authoritatively transferred to Globigerinoides based on genetic evidence by Morard et al. (2019a), allowing Globoturborotalita to be circumscribed as lacking supplementary apertures.
-
Globoturborotalita rubescens (Hofker, 1956)
Distinguished by the conspicuous reddish pigmentation of the shell, developed on most specimens and expressed in the species name. Hofker did not designate a holotype, neither in his original description of this species (Hofker, 1956), nor when describing the genus Globoturborotalita of which G. rubescens is the type species (Hofker, 1976). Hofker (1956) noted that the specimens that lead him to erect G. rubescens were derived from recent sediments from western Indonesia (Malay Archipelago) collected during the Dutch Siboga expedition (1899–1900). The location of this material is unclear, but we have located in the Hofker collection (originally deposited at the Zoological Museum in Amsterdam, and then transferred in 2011 to the Naturalis Biodiversity Center, Leiden, the Netherlands, and curated there) a slide registered as RGM 538508 with a written note on the slide apparently in Hofker's handwriting, stating that the sample contains “Globigerina rubescens Hofker” (Fig. 5c). The slide contains a sieved residue from a sediment collected during the Snellius expedition (Snellius station 190) off Sulawesi, i.e. from the same region as the Siboga material. It is impossible to establish when the labelling of the slide occurred, but because of the genus name it must have been before 1976. Since it appears likely that Hofker considered this material when he established G. rubescens in 1956 and because of the clear labelling by Hofker, the specimens in the sample can be considered syntypic, and the sample represents in our opinion the best available material to select a type. The residue contained a rich and well preserved assemblage of planktonic foraminifera from which we were able to extract 15 specimens of G. rubescens. From among those, we here designate specimen RGM 1333481 as the lectotype, and specimens RGM 1333482–1333495 as paralectotypes (Fig. 5).
-
Hastigerina Thomson in Murray, 1876
Monotypic genus without a notable fossil record, typified by Hastigerina pelagica (d'Orbigny, 1839c) representing a distinct lineage of spinose planktonic foraminifera with triradiate, barbed spines.
-
Hastigerina pelagica (d'Orbigny, 1839)
Banner and Blow (1960) selected a neotype, which is at the same time the lectotype of the junior synonym Hastigerina murrayi (Thompson, 1876). The species is genetically diverse (Weiner et al., 2012), and it may be that a closer examination of the three genetic lineages will justify taxonomic revision.
-
Hastigerinella Cushman, 1927
A substantial complexity arose due to the application of this genus on fossil taxa, which was clearly at variance with the intention of Cushman and the nature of the type species Hastigerinella digitata (Rhumbler, 1911). The genus was formally reinstated with Hastigerinella digitata as a type to stabilize the nomenclature of the Eocene Clavigerinella, by placing Hastigerinella digitata on the list of valid species by ICZN ruling (Coxall, 2003). In consequence, the replacement name Hastigerinellopsis Saito et al., 1976 had to be abandoned. To observe the ICZN ruling, this monotypic genus is retained, with the added advantage of providing a clear distinction between Beella digitata (Brady, 1879) and Hastigerinella digitata (Rhumbler, 1911).
-
Hastigerinella digitata (Rhumbler, 1911)
Following the opinion of Banner and Blow (1960b), who figured a number of specimens (hypotypes) and emended the species description in a way consistent with the current usage, the species has been formally placed on the list of valid taxa by ICZN ruling (Coxall, 2003). Banner and Blow (1960b) did not succeed in locating the type material, which is considered lost, but Banner (1965) later designated one of the hypotypes figured in Banner and Blow (1960b) as the neotype. Hastigerina parapelagica Saito and Thompson, 1976, is here considered a growth stage of Hastigerinella digitata, in a stage prior to the development of the branching chambers.
-
Neogallitellia Özdikmen, 2009
Monotypic genus established for the only extant species in the plankton with triserial chamber arrangement. A replacement name for Gallitellia Loeblich and Tappan, 1986, which is a junior homonym of the coral Gallitellia Cuif, 1977. One extant species.
-
Neogallitellia vivans (Cushman, 1934)
This small but distinct species, typified by a specimen from recent sediment, is frequently encountered in the plankton, especially along continental margins, and may be holoplanktonic, but its close genetic relationship with benthic taxa (Ujiié et al., 2008) implies that it could also represent a form that is planktonic only during one part of its life cycle, akin to the tychopelagic Bolivina variabilis.
-
Neogloboquadrina Bandy, Frerichs and Vincent, 1967
Typified by the extant Neogloboquadrina dutertrei (d'Orbigny, 1839a), this distinct and diverse genus has been established to separate the modern non-spinose lineage with “globoquadrinid” chambers from other fossil lineages possessing this character. The genus is paraphyletic, giving rise to Pulleniatina. Three extant and many fossil species.
-
Neogloboquadrina dutertrei (d'Orbigny, 1839)
A morphologically diverse tropical species, covering in its current concept specimens that have been in the past assigned to various taxa, most notably Neogloboquadrina eggeri (Rhumbler, 1901) or Neogloboquadrina blowi Rögl and Bolli, 1973, listed by Saito et al. (1981) despite the fact that Parker (1962) established a broad concept of the species, concluding that the different variants (four types described specifically) “all belong to the same species”. McCulloch (1977) alone described five new species, all being obvious synonyms of N. dutertrei. A lectotype showing morphological characteristics consistent with extant populations of the species was designated from recent sediments in the Caribbean by Banner and Blow (1960a). Kipp (1976) established the long-used informal category pachyderma/dutertrei (P/D) intergrade. There appears to exist only a limited amount of genetic diversity within the genus (André et al., 2014), providing so far no evidence justifying this category as a taxon. Most specimens assigned to this category are now considered N. incompta (Kucera et al., 2005).
-
Neogloboquadrina incompta (Cifelli, 1961)
Synonymized with N. pachyderma by Parker (1962), but reinstated by some workers and finally confirmed by the discovery of genetic distinction between the two coiling variants of Neogloboquadrina pachyderma by Darling et al. (2006). This species refers to specimens previously recorded in Quaternary sediments and plankton as N. pachyderma dextral (variously abbreviated) or N. pachyderma dextralis Setty, 1977, and likely comprise most specimens assigned to the pachyderma/dutertrei (P/D) intergrade of Kipp (1976). One of the six extant species with a holotype derived from the plankton.
-
Neogloboquadrina pachyderma (Ehrenberg, 1862)
Contrary to common belief, Ehrenberg's report on his investigations in 1861 of various deep-sea sediment samples was published in 1862, which must be considered the correct publication date for the species. The original specimen, later figured by Ehrenberg (1873), has been located and illustrated by Darling et al. (2006) and designated as type (as it comes from material which must be considered syntypic, it represents a lectotype). With N. incompta being accepted, the name N. pachyderma is reserved for specimens previously recorded in Quaternary sediments and plankton as N. pachyderma sinistral (variously abbreviated or incorrectly labelled as “sinistralis”, being a nomen nudum). Considerable morphological variability, noted already by Parker (1962), with numerous species names listed by Saito et al. (1981), is echoed by the existence of many distinct genetic types (Darling et al., 2017). In the absence of evidence for taxonomic significance of any of the alternative species concepts (or the many morphotypes in the species), all of the names previously applied to modern material are here considered synonyms. We note, however, that a future revision of the genetic variability and its link to shell morphology may lead to the reinstatement of some.
-
Orbulina d'Orbigny, 1826
Typified by the only extant species Orbulina universa d'Orbigny, 1839, Orbulina is together with Globigerina the earliest established genus of planktonic foraminifera. Several fossil species have been proposed but only O. universa and O. suturalis are currently recognized.
-
Orbulina universa d'Orbigny, 1839
Extant populations of Orbulina universa host specimens with shell morphologies that have been often identified as Orbulina suturalis and Biorbulina bilobata (e.g. Rossignol et al., 2011). However, despite earlier claims of the species surviving to the present (e.g. Kennett and Srinivasan, 1983; Aze et al., 2011), Orbulina suturalis is in our opinion to be understood as a short-lived species occurring in the middle Miocene (Wade et al. 2011) during a time when the diagnostic morphology of O. universa evolved (Pearson et al., 1997). Among living O. universa, forms similar to O. suturalis do occur but are rare and associated with an anomalously small spherical chamber relative to the enclosed “spiral” shell, or by the excentric envelopment of the sphere with respect to the spiral shell. Biorbulina bilobata is a rare form of O. universa referring to specimens that had formed a second terminal spherical chamber. This aberrant morphology is likely associated with higher food availability (Robbins, 1988), and its development has been observed in the laboratory during the growth of specimens that otherwise do not differ from Orbulina universa (Caron, 1987; Bonnin, et al., 2019). Consequently, O. suturalis and B. bilobata are not recognized as species or any other taxonomic categories among the living plankton. On the other hand, there is evidence that the three cryptic species of extant O. universa (de Vargas et al., 1999) are associated with differences in the size and distribution of pores and areal apertures on the terminal chamber (Morard et al., 2009), heralding a potential for future refinement of the taxonomy.
-
Orcadia Boltovskoy and Watanabe, 1982
The phylogenetic position of this monotypic genus remains enigmatic, requiring retention of the genus name (Holmes, 1984; Brummer et al., 1988c). It is here provisionally placed among the Hastigerinidae, awaiting revision based on molecular data.
-
Orcadia riedeli (Rögl and Bolli, 1973)
A conspicuous small species, occurring in temperate to subpolar waters of both hemispheres. The species is distinct and cannot be confused when observed under the SEM, but specimens in the plankton could be confused with T. quinqueloba or juvenile Hastigerina or Hastigerinella.
-
Pulleniatina Cushman, 1927
Typified by the extant Pulleniatina obliquiloculata (Parker and Jones, 1865), the concept of this genus remained stable since its establishment by Cushman (1927), referring to a distinct lineage descendant from Neogloboquadrina in the late Miocene. One extant and several fossil species.
-
Pulleniatina obliquiloculata (Parker and Jones, 1865)
Unusual among the extant planktonic foraminifera because of the streptospiral coiling of its adult chambers. The first mention of the name by Carpenter (1862) is considered a nomen nudum, and the correct publication year must be 1865. A neotype was selected by Bolli et al. (1957) from material in slide 94.4.3.1045 and figured and described by Banner and Blow (1960a, 1967). The material was initially considered syntypic, but Hodkinson (1992) pointed out that the material is not from the original syntype series and the type specimens must be considered to represent a neotype. Although not optimally preserved, the specimen does show the typical adult morphology. Numerous synonyms or names were applied to specific morphologies within the species (see Saito et al., 1981), most notably Pulleniatina finalis Banner and Blow, 1967, considered extant by Aze et al. (2011), and a host of names (Table 3) was applied to presumably immature specimens of the species with hispid shell surface, lacking the thick cortex. The species comprises three genetic types that appear separated longitudinally (Ujiié and Ishitani, 2016), but it is not clear if any of these are associated with morphological distinction.
-
Sphaeroidinella Cushman, 1927
Typified by the extant Sphaeroidinella dehiscens (Parker and Jones, 1865), the monotypic genus is justified by preserving common usage and paying tribute to the significance of the development of supplementary apertures as a genus-level character among all other Neogene lineages of planktonic foraminifera.
-
Sphaeroidinella dehiscens (Parker and Jones, 1865)
A rare but conspicuous extant species, characterized by a glassy cortex deposited at the end of its life cycle, but in pre-terminal stages found in the plankton resembling T. sacculifer (Huang, 1981). There is no evidence for more than one extant species and forms described as Sphaeroidinella excavata Banner and Blow, 1965, are considered as morphological variants without taxonomic significance.
-
Tenuitellita Li, 1987
Typified by the extant Globigerinita iota Parker, 1962, we propose this validly described but previously dismissed name to be used for the extant representatives of the microperforate lineage containing the type species instead of Tenuitella. This is because molecular phylogenetic analysis of the living representatives of the genus shows that they belong to a single clade derived relatively recently from Globigerinita (Morard et al., 2019b), and since the continuity of the modern species with the last surviving Miocene Tenuitella has not been established (Kennett and Srinivasan, 1983; Li, 1987), it is likely that the extant clade is unrelated and requires a different name. The existing and validly described Tenuitellita represents the obvious choice for a name based on one of the extant species, and we here also assign T. fleisheri and T. parkerae to this genus. Three extant species.
-
Tenuitellita fleisheri (Li, 1987)
Despite its distinct form, characterized by compressed chambers, and common occurrence in the fine fraction in the plankton, this species has often been ignored. Previously considered to be a junior synonym of T. compressa (Fordham, 1986), but Fordham's species has not been described validly and the name is not available. Schiebel and Hemleben (2017) list T. compressa alongside T. fleisheri, implying that they are distinct species, an opinion for which there is no morphological or genetic evidence. Indeed, one of the specimens of T. compressa pictured in Schiebel and Hemleben (2017, plate 2.35, fig. 4) is identical with an illustration in Hemleben et al. (1989), where the specimen is labelled as T. fleisheri. The earlier name by Fordham (1986) has to be rejected, because of the inconsistent use of binomial nomenclature in that work. The name “compressa” is labelled by Fordham (1986) as n.subsp. but also as n.ph. (ph. standing for phenon) of Todella grata (Todd, 1957), which, incidentally, Todd (1957) compared to Globorotalia mayeri, a distinct macroperforate taxon. Whereas a description as a subspecies may be valid even if the work where it is published does not follow binomial nomenclature (Article 11.4.2. of the Code), the concomitant designation of the taxon as n.ph. must be seen as a departure from the usage of Code categories or as an analogy to Article 15.2. of the Code and the name thus becomes unavailable.
-
Tenuitellita iota (Parker, 1962)
Described in detail by Parker (1962), the subsequent application of the species concept has been often hampered by the impression of the obligatory presence of a complex bulla, as seen on the type and two other specimens (plate 10, figs. 26–28 of Parker, 1962). This character refers to only one type within the species and Parker (1962) explicitly states that the species also contains a type where the bulla is rare, as exemplified by her specimen pictured on plate 20, fig. 29. In our experience, most specimens in the plankton have no terminal bulla but are easily distinguished from the superficially similar G. glutinata by their consistently umbilical–extraumbilical aperture and a distinctly smooth wall texture with few large pustules concentrated in the umbilical region, such as in the specimens figured as T. iota by Brummer (1988a) and Morard et al. (2019b).
-
Tenuitellita parkerae (Brönnimann and Resig, 1971)
Not to be confused with the homonymous Globigerinita parkerae (Bermúdez 1961). The latter refers to an extinct form (e.g. Kennett and Srinivasan, 1983), closely related to and perhaps even synonymous with G. glutinata. Tenuitellita parkerae is characterized by smooth wall texture and a tendency to grow radially elongated and slightly compressed chambers (Holmes, 1984; Brummer et al., 1988a), explaining why the species has been also assigned to the macroperforate Globorotalia (e.g. by Saito et al., 1981). Whilst the holotype represents the typical morphology, we note that the second specimen pictured by Brönniman and Resig (1971, their fig. 10) and designated as paratype is not microperforate and corresponds without doubt to the spinose Turborotalita clarkei.
-
Trilobatus Spezzaferri et al., 2015
Established by Spezzaferri et al. (2015), because both fossil and genetic evidence pointed to independent origin of this lineage from the clade including Globigerinoides ruber and allied species. The type species, Trilobatus trilobus (Reuss, 1850), is described from Miocene sediments, but its continuity with the extant species of the genus is well established (Spezzaferri et al., 2015). The genus is paraphyletic, giving rise to the Miocene Orbulina and Pliocene Globigerinoidesella (Poole and Wade, 2019). One extant and several fossil species and subspecies.
-
Trilobatus sacculifer (Brady, 1877)
The only extant representative of Trilobatus, shown to be genetically remarkably homogeneous (André et al., 2013), despite considerable morphological variability, leading to the proliferation of various names, which are all here rejected for the extant taxon (Table 3). In their concept of the species, Spezzaferri et al. (2015) distinguished late Oligocene and early Miocene populations of this lineage as Trilobatus trilobus (Reuss, 1850), lacking the typical sac-like final chamber, from late Neogene populations identified as T. sacculifer and consistently containing specimens with the sac-like final chamber. There is ample genetic (André et al., 2013) and fossil (Poole and Wade, 2019) evidence that the use of names other than T. sacculifer (such as T. trilobus, T. quadrilobatus, T. immaturus or any of the names describing particular forms of the final chamber such as T. hystricosus or Globigerinoidesella fistulosa) is inappropriate for the extant taxon, because there is no evidence for more than one species of Trilobatus at present. We note that some of those names may be valid for the description of fossil representatives of this lineage, with G. fistulosa representing a valid Pliocene form with distinct stratigraphic range (Poole and Wade, 2019). Since the sac-like final chamber is a facultative character, developed during the late ontogeny in some specimens (Bé et al., 1981), it cannot be used to distinguish taxa in the extant lineage even at the level of species. Therefore, we reject the usage of subspecies names in any combination, such as T. sacculifer trilobus or T. trilobus sacculifer, and advocate informal labelling of specimens with the sac-like final chamber, for example as T. sacculifer “with sac”. Beyond this distinction, the broad concept of the species is undisputed and likely to be applied consistently. The species is typified by an adequate, albeit likely fossil, lectotype selected by Banner and Blow (1960a) and re-illustrated and discussed by Williams et al. (2006).
-
Turborotalita Blow and Banner, 1962, emended Pearson and Kucera (2018)
A conspicuous genus of living planktonic foraminifera, typified by the extant Turborotalita humilis (Brady, 1884). In their emended diagnosis, Pearson and Kucera (2018) characterized the genus as having an adumbilically displaced, ampullate terminal chamber, which has been in the past inappropriately labelled as bulla. Three extant and several fossil species.
-
Turborotalita clarkei (Rögl and Bolli, 1973)
Although rarely recorded, T. clarkei likely represents the smallest as well as numerically most abundant of all modern species (Boltovskoy, 1991; Pearson et al., 2018; Chernikhovsky et al., 2020). Like T. humilis, T. clarkei is known in a lightly calcified lobate form commonly encountered in the living plankton as well as a heavily calcified compact (terminal) form often found in the sediment. The species was recognized by Saito et al. (1981) but erroneously assigned to Berggrenia. In Schiebel and Hemleben (2017), specimens of T. clarkei on plate 2.13, figs. 14–15 and plate 2.16, figs. 13–17 are erroneously labelled as T. quinqueloba.
-
Turborotalita humilis (Brady, 1884)
Like T. clarkei, T. humilis is known in a lightly calcified lobate form predominant in the living plankton as well as a heavily calcified and more compact (terminal) form commonly found in the sediment. Heavily calcified specimens with radially elongated chambers and lobate outline have also been referred to as Turborotalita cristata (Heron-Allen and Earland 1929), such as by Kennet and Srinivasan (1983) or Aze et al. (2011). Although the genetic diversity within T. humilis has not been sufficiently constrained yet, there is no evidence at present to consider T. cristata anything other than a calcification variant of T. humilis.
-
Turborotalita quinqueloba (Natland, 1938)
The morphological variability in this abundant species has led to the establishment of many names, proposed for terminal forms with differently developed ampullate final chambers (such as T. exumbilicata), including adult specimens from the living plankton without it (such as T. egelida), which we here subsume as ontogenetic variants under a single extant species. However, the species shows significant phenotypic variation (Kroon et al., 1988) and is genetically diverse (Darling et al., 2017) so that some of the names synonymized here may prove useful in future revisions considering pseudocryptic diversity, or when referring to extinct taxa.
No data sets were used in this article.
The supplement comprises an overview of all accepted species names with key distinguishing features (Supplement 1) and a list of species names (Supplement 2). The supplement related to this article is available online at: https://doi.org/10.5194/jm-41-29-2022-supplement.
The two authors contributed equally to the taxonomic research and writing of the paper.
The contact author has declared that neither they nor their co-author has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We appreciate the long-term support of our institutes in facilitating the painstakingly slow taxonomic work requiring frequent visits and consultations, as part of the long-standing MARUM-NIOZ partnership. This work was carried out within the framework of the SCOR/IGBP working group 138: Modern Planktonic Foraminifera and Ocean Changes. We are grateful to all working group members and affiliates for taxonomic discussions during various meetings. Paul Pearson and other members of the Paleogene Planktonic Foraminifera Working Group are acknowledged for extremely useful feedback on issues related to the congruency between the classification of living and fossil taxa. We thank the editor Sev Kender and all three reviewers for their critical comments, which improved the manuscript. Willem Renema and David Lazarus are thanked for their help with accessing museum collections. Brian Huber is thanked for providing SEM illustration of types from the Smithsonian Institution, and Adrian Baumeister is thanked for providing an illustration of a recent G. cultrata specimen. Raphael Morard is acknowledged for assistance with the figures, and Giles Miller and Madelaine Thompson are acknowledged for their assistance in identifying the location of William Beebe plankton material.
This research has been supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) through Germany's Excellence Strategy to the Cluster of Excellence “The Ocean Floor – Earth's Uncharted Interface” (EXC-2077 (grant no. 390741603)) and within the framework of the SCOR/IGBP working group 138: Modern Planktonic Foraminifera and Ocean Changes.
The article processing charges for this open-access publication were covered by the University of Bremen.
This paper was edited by Sev Kender and reviewed by Jeremy Young, Paul Pearson, and Bridget Wade.
Al-Sabouni, N., Fenton, I., Telford, R. J., and Kucera, M.: Reproducibility of species recognition in modern planktonic foraminifera and its implications for analyses of community structure, J. Micropalaeontol., 37, 519–534, 2018.
André, A., Weiner, A., Quillévéré, F., Aurahs, R., Morard, R., Douady, C. J., de Garidel-Thoron, T., Escarguel, G., de Vargas, C., and Kucera, M.: The cryptic and the apparent reversed: lack of genetic differentiation within the morphologically diverse plexus of the planktonic foraminifer Globigerinoides sacculifer, Paleobiology, 39, 21–39, 2013.
André, A., Quillévéré, F., Morard, R., Ujiié, Y., Escarguel, G., de Vargas, C., de Garidel-Thoron, T., and Douady, C. J.: SSU rDNA divergence in planktonic foraminifera: molecular taxonomy and biogeographic implications, PLoS ONE, 9, e104641, https://doi.org/10.1371/journal.pone.0104641, 2014.
Asano, K.: The foraminifera from the adjacent seas of Japan, collected by the S. S. Soyo-maru, 1922–1930: Part 3. Planktonic species, The science reports of the Tohoku University, Second series, Geology, 28, 1–26, 1957.
Aurahs, R., Göker, M., Grimm, G. W., Hemleben, V., Hemleben, C., Schiebel, R., and Kučera, M.: Using the multiple analysis approach to reconstruct phylogenetic relationships among planktonic foraminifera from highly divergent and length-polymorphic SSU rDNA sequences, Bioinform. Biol. Insights, 3, 155–177, 2009.
Aurahs, R., Treis, Y., Darling, K. F., and Kucera, M.: A revised taxonomic and phylogenetic concept for the planktonic foraminifer species Globigerinoides ruber based on molecular and morphometric evidence, Mar. Micropalaeontol., 79, 1–14, 2011.
Aze, T., Ezard, T. H. G., Purvis, A., Coxall, H. K., Stewart, D. R. M., Wade, B. S., and Pearson, P. N. P.: A phylogeny of Cenozoic macroperforate planktonic foraminifera from fossil data, Biol. Rev., 86, 900–927, 2011.
Bandy, O. L., Frerichs, W. E., and Vincent, E.: Origin, development, and geologic significance of Neogloboquadrina Bandy, Frerichs, and Vincent, gen. nov, Cushman Found. Foram. Res. Contr., 18, 152–157, 1967.
Banner, F. T.: On Hastigerinella digitata (Rhumbler, 1911), Micropaleontology, 11, 114–116, 1965.
Banner, F. T. and Blow, W. H.: The classification and stratigraphic distribution of the Globigerinacea, Paleontology, 2, 1–27, 1959.
Banner, F. T. and Blow, W. H.: Some primary types of species belonging to the superfamily Globigerinaceae, Cushman Found. Foram. Res. Contr., 11, 1–41, 1960a.
Banner, F. T. and Blow, W. H.: The taxonomy, morphology and affinities of the genera included in the subfamily Hastigerininae, Micropaleontology, 6, 19–31, 1960b.
Banner, F. T. and Blow, W. H. Progress in the planktonic foraminiferal biostratigraphy of the Neocene, Nature, 208, 1164–1166, 1965.
Banner, F. T. and Blow, W. H.: The origin, evolution and taxonomy of the foraminiferal genus Pulleniatina Cushman, 1927, Micropaleontology, 13, 133–162, 1967.
Barraclough, T. G.: The evolutionary biology of species, Oxford, Oxford University Press, https://doi.org/10.1093/oso/9780198749745.001.0001, 2019.
Bé, A. W. H.: Foraminifera families: Globigerinidae and Globorotaliidae, Zooplankton, Fiches d'Indentification du Zooplancton, Cons. Perm. Intern. Eplor. de la Mer, Sheet 108, Charlottenlund Slot, Danemark, 1–9, 1967a.
Bé, A. W. H.: Globorotalia cavernula, a new species of planktonic foraminifera from the Subantarctic Pacific Ocean, Cushman Found. Foram. Res. Contr., 18, 128–132, 1967b.
Bé, A. W. H.: An ecological, zoogeographic and taxonomic review of Recent planktonic foraminifera, in: Oceanic Micropaleontology, Volume 1, edited by: Ramsey, A. T. S., Academic Press, London, 1–100, 1977.
Bé, A. W. H., Caron, D. A., and Anderson, O. R.: Effects of feeding frequency on life processes of the planktonic foraminifer Globigerinoides sacculifer in laboratory culture, J. Mar. Biol. Assoc. U.K., 61, 257–277, 1981.
Bermudez, P. J.: Contribution al estudio de las Globigerinidea de la region Caribe-Antillana (Paleoceno-Reciente), Dir. Geol., Bol. Geol. Publ. Espec., 3 (Congr. Geol. Venezolano, III, 1960, Mem.), 1119–1393, 1960.
Bermúdez, P. J. and Bolli, H. M.: Consideraciones sobre los sedimentos del Mioceno medio al Reciente de las costas central y oriental de Venezuela, Bol. Geol., Dir. Geol., Minister. Minas Hidrocarb., 10, 137–223, 1969.
Blow, W. H.: Age, correlation, and biostratigraphy of the upper Tocuyo (San Lorenzo) and Pozon Formations, eastern Falcon, Venezuela, B. Am. Paleontol., 39, 67–251, 1959.
Blow, W. H.: Late middle Eocene to Recent planktonic foraminiferal biostratigraphy, in: Proc. First Int. Conf. Planktonic Microfossils, Geneva, 1967, Vol. 1, edited by: Brönnimann, P. and Renz, H. H., Leiden, E.J. Brill, 199–422, 1969.
Blow, W. H. and Banner, F. T.: The mid-Tertiary (Upper Eocene to Aquitanian) Globigerinaceae, in: Fundamentals of mid-Tertiary Stratigraphical Correlation, edited by: Eames, F. E., Banner, F. T., Blow, W. H., and Clarke, W. J., Cambridge University Press, Cambridge, 61–151, 1962.
Bohrmann, G., Lahajnar, N., Gaye, B., Spieß, V., and Betzler, C.: Nitrogen cycle, cold seeps, carbonate platform development in the Northwestern Indian Ocean, Cruise No. 74, 31 August–22 December 2007, METEOR-Berichte, Leitstelle Meteor, Institut für Meereskunde der Universität Hamburg, 10-3, 1–212, https://doi.org/10.2312/cr_m74, 2010.
Bolli, H. M.: Planktonic foraminifera from the Oligocene-Miocene Cipero and Lengua formations of Trinidad, B. W. I., in: Studies in Foraminifera, edited by: Loeblich Jr., A. R., Tappan, H., Beckmann, J. P., Bolli, H. M., Montanaro Gallitelli, E., and Troelsen, J. C., U.S. Natl. Museum Bull., 215, 97–123, 1957.
Bolli, H. M., Loeblich, A. R., and Tappan, H.: Planktonic foraminiferal families Hantkeninidae, Orbulinidae, Globorotaliidae and Globotruncanidae, in: Studies in Foraminifera, edited by: Loeblich Jr., A. R., Tappan, H., Beckmann, J. P., Bolli, H. M., Montanaro Gallitelli, E., and Troelsen, J. C., U.S. Natl. Museum Bull., 215, 3–50, 1957.
Boltovskoy, E.: Globorotalia hirsuta eastropacia n. subsp. – planktonic subspecies (Foraminiferida) from the tropical Pacific Ocean, Rev. Esp. Micropaleontol., 6, 127–133, 1974.
Boltovskoy, E.: Globigerinita clarkei (Rögl & Bolli) – an unfairly ignored small planktic foraminifer, Boreas, 20, 151–154, 1991.
Boltovskoy, E.: Living planktonic foraminifera of the eastern part of the tropical Atlantic, Rev. Micropal., 11, 85–98, ISSN 0035-1598, 1968.
Boltovskoy, E. and Watanabe, S.: Orcadia, nuevo genero de Foraminiferos planctonicos Antarticos, Rev. Esp. Micropaleontol., 14, 5–11, 1982.
Bonnin, E. A., Zhu, Z., Fehrenbacher, J. S., Russell, A. D., Hönisch, B., Spero, H. J., and Gagnon, A. C.: Submicron sodium banding in cultured planktic foraminifera shells, Geochim. Cosmochim. Ac., 253, 127–141, 2019.
Brady, H. B.: Supplementary note on the foraminifera of the Chalk (?) of the New Britain group, Geol. Mag., 4, 534–536, https://doi.org/10.1017/S0016756800150137, 1877.
Brady, H. B.: Notes on some of the reticularian Rhizopoda of the “Challenger” expedition. I. – On new or little known arenaceous types, Q. J. Micros. Sci., 19, 20–63, 1879.
Brady, H. B.: Report on the Foraminifera, in: Exploration of the Faroe Channel, during the summer of 1880, in: H.M.S. “Knight Errant”, with subsidiary reports, edited by Tizard, Murray, J., P. Roy. Soc. Edinb., 11, 708–717, 1882.
Brady, H. B.: Report on the Foraminifera dredged by H.M.S. Challenger during the years 1873–1876, Report on the Scientific Results of the Voyage of H.M.S. Challenger during the year 1873–1876, Zoology, 9, 1–814, 1884.
Brönnimann, P.: Globigerinita naparimaensis n. gen., n. sp., from the Miocene of Trinidad, B. W. I., Cushman Found. Foram. Res. Contr., 2, 16–18, 1951.
Brönnimann, P. and Resig, J.: A Neogene globigerinacean biochronologic time-scale of the southwestern Pacific, Initial Rep. Deep Sea, 7, 1235–1469, 1971.
Brummer, G. J. A., Hemleben, C., and Spindler, M.: Planktonic foraminiferal ontogeny and new perspectives for micropalaeontology, Nature, 319, 50–52, 1986.
Brummer, G. J. A.: Comparative ontogeny of modern microperforate planktonic foraminifers, in: Planktonic foraminifers as tracers of ocean-climate history, edited by: Brummer, G. J. A. and Kroon, D., Free University Press, Amsterdam, 77–129, 1988a.
Brummer, G. J. A.: Comparative ontogeny and species definition of planktonic foraminfers: A case study of Dentigloborotalia anfracta n. gen., in: Planktonic foraminifers as tracers of ocean-climate history, edited by: Brummer, G. J. A. and Kroon, D., Free University Press, Amsterdam, 51–75, 1988b.
Brummer, G. J. A., Troelstra, S. R., Kroon, D., and Ganssen, G. M.: Ontogeny, distribution and geologic record of the extant planktonic foraminifer Orcadia riedeli (Rögl & Bolli, 1973), in: Planktonic foraminifers as tracers of ocean-climate history, edited by: Brummer, G. J. A. and Kroon, D., Free University Press, Amsterdam, 149–162, 1988c.
Brummer, G.-J. A., Metcalfe, B., Feldmeijer, W., Prins, M. A., van 't Hoff, J., and Ganssen, G. M.: Modal shift in North Atlantic seasonality during the last deglaciation, Clim. Past, 16, 265–282, https://doi.org/10.5194/cp-16-265-2020, 2020.
Buckley, H. A.: Globorotalia (Clavatorella) oveyi n. sp., premiere mention Récente d'un sous-genre de Foraminifere du Neogene, Rev. Micropaléontol., 16, 168–172, 1973.
Burckhardt, G.: Zum Worte Plankton, Schweiz. Z. Hydrol, 1, 190–192, 1920.
Caron, D. A., Faber, W. W., and Bé, A. W.: Growth of the spinose planktonic foraminifer Orbulina universa in laboratory culture and the effect of temperature on life processes, J. Mar. Biol. Assoc. UK, 67, 343–358, 1987.
Carpenter, W. B.: Introduction to the study of the Foraminifera, published for the Ray Society by Robert Hardwicke, 1–319, 1862.
Chapman, F., Parr, W. J., and Collins, A. C.: Tertiary foraminifera of Victoria, Australia – The Balcombian deposits of Port Phillip, part III, Zool. J. Linn. Soc.-Lond., 38, 553–577, 1934.
Chernihovsky, N., Almogi-Labin, A., Kienast, S. S., and Torfstein, A.: The daily resolved temperature dependence and structure of planktonic foraminifera blooms, Sci. Rep.-UK, 10, 1–12, 2020.
Cifelli, R.: Globigerina incompta, a new species of pelagic foraminifera from the North Atlantic, Cushman Found. Foram. Res. Contr., 12, 83–86, 1961.
CLIMAP Project Members: The surface of the ice-age Earth, Science, 191, 1131–1137, 1976.
Coxall, H. K.: Hastigerinella Cushman, 1927 and Clavigerinella Bolli, Loeblich & Tappan, 1957 (Rhizopodea, Foraminiferida); proposed conservation of the usage by designation of Hastigerina digitata Rhumbler, 1911 as the type species of Hastigerinella, Bull. Zool. Nomenclature, 60, 182–186, 2003.
Coxall, H. K. and Spezzaferri, S.: Taxonomy, biostratigraphy, and phylogeny of Oligocene Catapysdrax, Globortaloides and Protentelloides, in: Atlas of Oligocene Planktonic Foraminifera, edited by: Wade, B. S., Olsson, R. K., Pearson, P. N., Huber, B. T., and Berggren, W. A., Cushman Found. Foram. Res. Spec. Publ., 46, 79–124, 2018.
Cuif, J. P.: Caracteres et affinites de Gallitellia, nouveau genre de madreporaires du ladino-carnien des Dolomites, 2eme Congr. Int. Cnidaires fossiles, Paris, Mem. Bur. Rech. Geol. Min., 89, 256–263, 1977.
Cushman, J. A.: An outline of a reclassification of the foraminifera, Contributions from the Cushman Laboratory for Foraminiferal Research, Contr. Cushman Lab. Foram. Res., 3, 1–105, 1927.
Cushman, J. A.: A Recent Guembelitria (?) from the Pacific, Contr. Cushman Lab. Foram. Res., 10, 105–106, 1934.
Cushman, J. A. and Bermúdez, P. J.: Some Cuban species of Globorotalia, Contr. Cushman Lab. Foram. Res., 25, 26–45, 1949.
d'Orbigny, A. D.: Tableau methodique de la classe des Cephalopodes, Ann. Sci. Nat., 1, 245–314, 1826.
d'Orbigny, A. D.: Foraminiferes, in: Histoire physique et naturelle de l'Ile de Cuba, edited by: de la Sagra, R. and Bertrand, A., Paris, 1–224, 1839a.
d'Orbigny, A. D.: Foraminifères des Iles Canaries, in: Histoire naturelle des Iles Canaries, edited by: Barker-Webb, P. and Berthelot, S., 120–146, 1839b.
d'Orbigny, A. D.: Voyage dans l'Amérique Méridionale, Foraminifères, 5, 1–86, 1839c.
Darling, K. F. and Wade, C. M.: The genetic diversity of planktic foraminifera and the global distribution of ribosomal RNA genotypes, Mar. Micropaleontol., 67, 216–238, 2008.
Darling, K. F., Kucera, M., Kroon, D., and Wade, C. M., A resolution for the coiling direction paradox in Neogloboquadrina pachyderma, Paleoceanography, 21, PA2011, https://doi.org/10.1029/2005PA001189, 2006.
Darling, K. F., Thomas, E., Kasemann, S. A., Seears, H. A., Smart, C. W., and Wade, C. M.: Surviving mass extinction by bridging the benthic/planktic divide, P. Natl. Acad. Sci. USA, 106, 12629–12633, 2009.
Darling, K. F., Wade, C. M., Siccha, M., Trommer, G., Schulz, H., Abdolalipour, S., and Kurasawa, A.: Genetic diversity and ecology of the planktonic foraminifers Globigerina bulloides, Turborotalita quinqueloba and Neogloboquadrina pachyderma off the Oman margin during the late SW Monsoon, Mar. Micropaleontol., 137, 64–77, 2017.
De Klasz, I., Kroon, D., and Van Hinte, J. E.: Notes on the foraminiferal genera Laterostomella de Klasz and Rerat and Streptochilus Brönnimann and Resig, J. Micropalaeontol., 8, 215–225, 1989.
de Vargas, C., Norris, R., Zaninetti, L., Gibb, S. W., and Pawlowski, J.: Molecular evidence of cryptic speciation in planktonic foraminifers and their relation to oceanic provinces, P. Natl. Acad. Sci. USA, 96, 2864–2868, 1999.
Earland, A.: Foraminifera, Part III, The Falklands sector of the Antarctic (excluding South Georgia), Discovery Reports, 10, 1–208, 1934.
Egger, J. G.: Foraminiferen aus Meeresgrundproben, gelothet von 1874 bis 1876 von S. M. Sch. Gazelle, Abh. K. Bayer. Akad. Wiss., Cl. II, 18, 195–457, 1893.
Ehrenberg, C. G.: Elemente des tiefen Meeresgrundes in Mexikanischen Golfstrome bei Florida; Über die Tiefgrund-Verhältnisse des Oceans am Eingange der Davisstrasse und bei Island, K. Preuss. Akad. Wiss. Berlin, 1861, 275–315, 1862.
Ehrenberg, C. G.: Mikrogeologische Studien über das kleinste Leben der Meeres-Tiefgründe aller Zonen und dessen geologischen Einfluss, K. Preuss. Akad. Wiss. Berlin, Abh., Physikalische Klasse, 1872, 131–398, 1873.
Emiliani, C.: Planktic/planktonic nektic/nektonic; benthic/benthonic, J. Paleontol., 65, p. 329, 1991a.
Emiliani, C.: Planktic et al., Mar. Micropaleontol., 18, p. 3, 1991b.
Faber, W. W., Anderson, O. R., Lindsey, J. L., and Caron, D. A.: Algal-foraminiferal symbiosis in the planktonic foraminifer Globigerinella aequilateralis, I, Occurrence and stability of two mutually exclusive chrysophyte endosymbionts and their ultrastructure, J. Foramin. Res., 18, 334–343, 1988.
Fenton, I. S., Baranowski, U., Boscolo-Galazzo, F., Cheales, H., Fox, L., King, D. J., Larkin, C., Latas, M., Liebrand, D., Miller, C. G., Nilsson-Kerr, K., Piga, E., Pugh, H., Remmelzwaal, S., Roseby, Z. A., Smith, Y. M., Stukins, S., Taylor, B., Woodhouse, A., Worne, S., Pearson, P. N., Poole, C. R., Wade, B. S., and Purvis, A.: Factors affecting consistency and accuracy in identifying modern macroperforate planktonic foraminifera, J. Micropalaeontol., 37, 431–443, 2018.
Finlay, H. J.: New Zealand foraminifera: Key species in stratigraphy - no. 5, New Zeal. J. Sci. Tech., 28, 259–292, 1947.
Fleisher, R. L.: Cenozoic planktonic foraminifera and biostratigraphy, Arabian Sea, Deep Sea Drilling Project, Leg 23A, Initial Rep. Deep Sea, 23, 1001–1072, 1974.
Fordham, B. G.: Miocene–Pleistocene planktic foraminifers from D. S. D. P. Sites 208 and 77, and phylogeny and classification of Cenozoic species, Evol. Monogr., 6, 1–200, 1986.
Fordham, B. G.: Toddina, replacement name for Toddella Fordham, 1986 (type species, “Globigerina? grata Todd, 1957”), J. Foramin. Res., 18, p. 84, 1988.
Galloway, J. J. and Wissler, S. G.: Pleistocene foraminifera from the Lomita Quarry, Palos Verdes Hills, California, J. Paleontol., 1, 35–87, 1927.
Ganssen, G. M. and Kucera, M.: SCOR/IGBP working group on modern planktonic foraminifera kicked off, PAGES news, 20, p. 6, 2012.
GBIF.org: GBIF Home Page, https://www.gbif.org, last access: 13 January 2020.
Haman, D.: Globigerinita iota Parker, 1962, and the validity of Tenuitellita Li, 1987 (Foraminiferida), J. Micropalaeontol., 7, 241–242, https://doi.org/10.1144/jm.7.2.241, 1988.
Hayward, B. W., Le Coze, F., Vachard, D., and Gross, O.: World Foraminifera Database, https://doi.org/10.14284/305, http://www.marinespecies.org/foraminifera, last access: 4 April 2020.
Hemleben, C., Spindler, M., and Anderson, O. R.: Modern planktonic foraminifera, Springer, New York, Berlin, Heidelberg, 1–363, 1989.
Henderson, G. M.: New oceanic proxies for paleoclimate, Earth Planet. Sc. Lett., 203, 1–13, 2002.
Heron-Allen, A. and Earland, A.: Some new foraminifera from the South Atlantic II, J. R.l Micros. Soc., 49, 324–334, 1929.
Hesemann, M.: The foraminifera.eu database: concept and status, Palaeontol. Electr., 18.3.48A, 1-14, 2015.
Hsiang, A. Y., Brombacher, A., Rillo, M. C., Mleneck-Vautravers, M. J., Conn, S., Lordsmith, S., Jentzen, A., Henehan, M. J., Metcalfe, B., Fenton, I. S., Wade, B. S., Fox, L., Meilland, J., Davis, C. V., Baranowski, U., Groeneveld, J., Edgar, K. M., Movellan, A., Aze, T., Dowsett, H. J., Miller, C. G., Rios, N., and Hull, P. M.: Endless forams: >34,000 modern planktonic foraminiferal images for taxonomic training and automated species recognition using convolutional neural networks, Paleoceanogr. Paleocl., 34, 1157–1177, 2019.
Hodgkinson, R. L.: W. K. Parker's collection of foraminifera in the British Museum (Natural History). Bulletin of the British Museum (Natural History), Geology, 48, 45–78, 1992.
Hofker, J.: Morphology of Globigerinatella insueta Cushman and Stainforth, Cushman Found. Foram. Res. Contr., 5, 151–152, 1954.
Hofker, J.: Foraminifera Dentata, foraminifera of Santa Cruz and Thatch Island, Virgin Archipelago, West Indies, Spolia Zool. Mus. Kobenhaven, 15, 1–237, 1956.
Hofker, J.: La familie Turborotaliidae n. fam., Rev. Micropaleontol., 19, 47–53, 1976.
Holmes, N. A.: An emendation of the genera Beella Banner and Blow, 1960, and Turborotalita Blow and Banner, 1962; with notes on Orcadia Boltovskoy and Watanabe, 1982, J. Foramin. Res., 14, 101–110, 1984.
Huang, C. Y.: Observations on the interior of some late Neogene planktonic foraminifera, J. Foramin. Res., 11, 173–190, 1981.
Huang, T.: Alloglobigerinoides, a new planktic foraminiferal genus, Petrol. Geol. Taiwan, 22, 93–102, 1986.
Huber, B. T., Olsson, R. K., and Pearson, P. N.: Taxonomy, biostratigraphy, and phylogeny of Eocene microperforate planktonic foraminifera (Jenkinsina, Cassigerinelloita, Chiloguembelina, Streptochilus, Zeauvigerina, Tenuitella, and Cassigerinella) and Problematica (Dipsidripella), in: Atlas of Eocene Planktonic Foraminifera, edited by: Pearson, P. N., Olsson, R. K., Hemleben, C., Huber, B. T., and Berggren, W. A., Cushman Found. Foramin. Res. Sp. Publ., 41, 461–508, 2006.
Huber, B. T., Petrizzo, M. R., Young, J. R., Falzoni, F., Gilardoni, S., Bown, P. R., and Wade, B.: Pforams@mikrotax: A new online taxonomic database for planktonic foraminifera, Micropaleontology, 62, 429–438, 2016.
Hutchinson, G. E.: De rebus planctonicis, Limnol. Oceanogr., 19, 360–361, 1974.
ICZN: Opinion 1234 Rotalia menardii Parker, Jones & Brady, 1865 (Foraminiferida): Neotype designated, Bull. Zool. Nomenclature, 39, 253–254, 1982.
Jonkers, L. and Kučera, M.: Quantifying the effect of seasonal and vertical habitat tracking on planktonic foraminifera proxies, Clim. Past, 13, 573–586, https://doi.org/10.5194/cp-13-573-2017, 2017.
Jonkers, L., Cartapanis, O., Langner, M., McKay, N., Mulitza, S., Strack, A., and Kucera, M.: Integrating palaeoclimate time series with rich metadata for uncertainty modelling: strategy and documentation of the PALMOD 130k marine palaeoclimate data synthesis, Earth Syst. Sci. Data, 12, 1053–1081, 2020.
Kipp, N. G.: New transfer function for estimating past sea-surface conditions from sea-bed distribution of planktonic foraminiferal assemblages in the North Atlantic, in: Investigations of Late Quaternary paleoceanography and paleoclimatology, edited by: Cline R. M. and Hays, J. D., Mem. Geol. Soc. Am., 145, 3–41, 1976.
Kennett, J. P. and Srinivasan, M. S.: Neogene Planktonic Foraminifera, Hutchinson Ross Publishing Co., Stroudsburg, Pennsylvania, 1–265, ISBN 10 0879330708, ISBN 13 978-0879330705, 1983.
Koch, R.: Die jungtertiare Foraminiferenfauna von Kabu (Res. Surabaja Java), Eclogae Geol. Helv., 18, 342–357, 1923.
Kroon, D. and Nederbragt, A. J.: Ecology and paleoecology of triserial planktic foraminifera, Mar. Micropaleontol., 16, 25–38, 1990.
Kroon, D., Wouters, P. F., Moodley, L., Ganssen, G., and Troelstra, S. R.: Phenotypic variation of Turborotalita quinqueloba (Natland) tests in living populations and in the Pleistocene of an eastern Mediterranean piston core. in: Planktonic foraminifers as tracers of ocean-climate history, edited by: Brummer, G. J. A. and Kroon, D., Free University Press, Amsterdam, 131–147, 1988.
Kucera, M.: Planktonic foraminifera as tracers of past oceanic environments, in: Developments in Marine Geology, Volume 1, Proxies in late Cenozoic Paleoceanography, edited by: Hillaire-Marcel, C. and de Vernal, A., Elsevier, 213–262, https://doi.org/10.1016/S1572-5480(07)01011-1, 2007.
Kucera, M., Weinelt, Mara, Kiefer, T., Pflaumann, U., Hayes, A., Weinelt, Martin, Chen, M.-T., Mix, A. C., Barrows, T. T., Cortijo, E., Duprat, J., Juggins, S., and Waelbroeck, C.: Reconstruction of sea-surface temperatures from assemblages of planktonic foraminifera: Multi-technique approach based on geographically constrained calibration datasets and its application to glacial Atlantic and Pacific Oceans, Quaternary Sci. Rev., 24, 951–998, 2005.
Kucera, M., Sylie, L., Weiner, A., Darling, K. F., Lübben, B., Holzmann, M., Pawlowski, J., Schönfeld, J., and Morard, R.: Caught in the act: Anatomy of an ongoing benthic-planktonic transition in a marine protest, J. Plankton Res., 39, 436–449, 2017.
Lamb, J. L. and Beard, J. H.: Late Neogene planktonic foraminifers in the Caribbean, Gulf of Mexico, and Italian stratotypes, The University of Kansas Paleontological Contributions, Article 57 (Protozoa 8), 1–67, Elsevier, https://doi.org/10.1016/S1572-5480(07)01011-1, 1972.
Le Calvez, Y.: Révision des Foraminifères de la collection d'Orbigny 1. Foraminifères des iles Canaries, Cah. Micropal., 2, 1–107, 1974.
Leckie, R. M., Wade, B. S., Pearson, P. N., Fraass, A. J., King, D. J., Olsson, R. K., Premoli Silva, I., Spezzaferri, S., and Berggren, W. A.: Taxonomy, biostratigraphy, and phylogeny of Oligocene and early Miocene Paragloborotalia and Parasubbotina, in: Atlas of Oligocene Planktonic Foraminifera, edited by: Wade, B. S., Olsson, R. K., Pearson, P. N., Huber, B. T., and Berggren, W. A., Cushman Found. Foramin. Res. Sp. Publ., 46, 125–178, 2018.
Li, Q.: Origin, phylogenetic development and systematic taxonomy of the Tenuitella plexus (Globigerinitidae, Globigerininina), J. Foramin. Res., 17, 298–320, 1987.
Lipps, J. H., Finger, K. L., and Walker, S. E.: What should we call the foraminifera?, J. Foramin. Res., 41, 309–313, 2011.
Lirer, F., Foresi, L. M., Iaccarino, S. M., Salvatorini, G., Turco, E., Cosentino, C., Sierro, F. J., and Caruso, A.: Mediterranean Neogene planktonic foraminifer biozonation and biochronology, Earth-Sci. Rev., 196, 102869, https://doi.org/10.1016/j.earscirev.2019.05.013, 2019.
Loeblich Jr., A. R. and Tappan, H.: The new planktonic foraminiferal genus Tinophodella and an emendation of Globigerinita Bronnimann, J. Washington Acad. Sci., 47, 112–116, 1957.
Loeblich Jr., A. R. and Tappan, H.: Suprageneric classification of the Foraminiferida (Protozoa), Micropaleontology, 30, 1–70, 1984.
Loeblich Jr., A. R. and Tappan, H.: Some New and Revised Genera and Families of Hyaline Calcareous Foraminiferida (Protozoa), Trans. Amer. Microscopical Soc., 105, 239–265, https://doi.org/10.2307/3226297, 1986.
Loeblich Jr., A. R. and Tappan, H.: Foraminiferal General and Their Classification, Van Nostrand Reinhold Company, New York, 1–970, ISBN 978-1-4899-5760-3, 1988.
Lohmann, G. P. and Malmgren, B. A.: Equatorward migration of Globorotalia truncatulinoides ecophenotypes through the Late Pleistocene: gradual evolution or ocean change?, Paleobiology, 9, 414–421, 1983.
Malmgren, B. A. and Kennett, J. P.: Biometric differentiation between Recent Globigerina bulloides and Globigerina falconensis in the southern Indian Ocean, J. Foramin. Res., 7, 131–148, 1977.
Martinsson, A.: Editor's column: Planktic, nektic, benthic, Lethaia, 8, 193–194, 1975.
Martinsson, A.: Planktic versus planktonic once more, Lethaia, 12, p. 244, 1979.
Martinsson, A.: How to retain planktic organisms and escape Platic love, Lethaia, 15, p. 30, 1982.
McCulloch, I.: Qualitative observations on Recent foraminiferal tests with emphasis on the eastern Pacific, University of Southern California, Los Angeles, OCLC no. 869277273, 1977.
Mitra, R., Marchitto, T. M., Ge, Q., Zhong, B., Kanakiya, B., Cook, M. S., Fehrenbacher, J. S., Ortiz, J. D., Tripati, A., and Lobaton, E.: Automated species-level identification of planktic foraminifera using convolutional neural networks, with comparison to human performance, Mar. Micropaleontol., 147, 16–24, 2019.
Morard, R., Quillévéré, F., Escarguel, G., Ujiie, Y., de Garidel-Thoron, T., Norris, R. D., and de Vargas, C.: Morphological recognition of cryptic species in the planktonic foraminifer Orbulina universa, Mar. Micropaleontol., 71, 148–165, 2009.
Morard, R., Quillévéré, F., Douady, C. J., de Vargas, C., de Garidel-Thoron, T., and Escarguel, G.: Worldwide genotyping in the planktonic foraminifer Globoconella inflata: Implications for life history and paleoceanography, PLoS ONE, 6, e26665, https://doi.org/10.1371/journal.pone.0026665, 2011.
Morard, R., Darling, K., Mahé, F., Audic, S., Ujiié, Y., Weiner, A., André, A., Seears, H., Wade, C., Quillévéré, F., Douady, C., Escarguel, G., de Garidel-Thoron, T., Siccha, M., Kucera, M., and de Vargas, C.: PFR2: a curated database of planktonic Foraminifera 18S ribosomal DNA as a resource for studies of plankton ecology, biogeography, and evolution, Mol. Ecol. Resour., 15, 1472–1485, 2015.
Morard, R., Escarguel, G., Weiner, A., André, A., Douady, C. J., Wade, C. M., Darling, K. F., Ujiié, Y., Seears, H. A., Quillévéré, F., de Garidel-Thoron, T., de Vargas, C., and Kucera, M.: Nomenclature for the nameless: a proposal for an integrative molecular taxonomy of cryptic diversity exemplified by planktonic foraminifera, Systematic Biol., 65, 925–940, 2016.
Morard, R., Füllberg, A., Brummer, G. J. A., Greco, M., Jonkers, L., Wizemann, A., Weiner, A. K. M., Darling, K. F., Siccha, M., Ledevin, R., Kitazato, H., de Garidel-Thoron, T., de Vargas, C., and Kucera, M.: Genetic and morphological divergence in the warm-water planktonic foraminifera genus Globigerinoides, PLoS ONE, 14, e0225246, https://doi.org/10.1371/journal.pone.0225246, 2019a.
Morard, R., Vollmar, N. M., Greco, M., and Kucera, M.: Unassigned diversity of planktonic foraminifera from environmental sequencing revealed as known but neglected species, PLoS ONE, 14, 1–18, 2019b.
Murray, J.: Preliminary reports to Professor Wyville Thomson, F. R. S., director of the Civilian Scientific Staff, on work done on board the “Challenger”, P. Roy. Soc. Lond., 24, 471–544, 1876.
Natland, M. L.: New Species of Foraminifera from off the West Coast of North America and from the Later Tertiary of the Los Angeles Basin, B. Scripps Inst. Oceanogr., Tech. Ser., 4, 137–164, 1938.
Notice of New Applications to the Commission (Case 3827–3838), Bull. zool. nomencl., 77, 113–114, 2020.
Olsson, R. K., Hemleben, C., Berggren, W. A., and Huber, B. T.: Atlas of Paleocene planktonic foraminifera, SM. C. Paleob., 85, 1–252, 1999.
Özdikmen, H.: Substitute names for some unicellular animal taxa (Protozoa), Munis Entomol. Zool., 4, 233–256, 2009.
Parker, F. L.: Eastern Mediterranean foraminifera, Reports of the Swedish Deep Sea Expedition 1947–1948, 8, 217–283, 1958.
Parker, F. L.: Planktonic foraminifera species in Pacific sediments, Micropaleontology, 8, 219–254, 1962.
Parker, F. L.: Late Tertiary biostratigraphy (planktonic foraminifera) of tropical Indo-Pacific deep-sea cores, Bull. Am. Paleontol., 52, 114–208, 1967.
Parker, F. L.: Taxonomic notes on some planktonic Foraminifera, in: Progress in micropaleontology, edited by: Takayanagi, Y. and Saito, T., Micropaleontology Press, New York, 258–262, 1976.
Parker, W. K. and Jones, T. R.: On some foraminifera from the North Atlantic and Arctic Oceans, including Davis Straits and Baffin's Bay, Philos. T. R. Soc. Lond., 155, 325–441, 1865.
Parker, W. K., Jones, T. R., and Brady, H. B.: On the nomenclature of the foraminifera. XII: The Species enumerated by d'Orbigny in the “Annales des Sciences Naturelles”, Vol. 7, 1826, Ann. Mag. Nat. Hist., 3, 15–41, 1865.
Pearson, P. N.: Oxygen Isotopes in Foraminifera: Overview and Historical Review, The Paleontological Society Papers, 18, 1–38, 2012.
Pearson, P. N. and IODP Expedition 363 Shipboard Scientific Party: A deep-sea agglutinated foraminifer tube constructed with planktonic foraminifer shells of a single species, J. Micropalaeontol., 37, 97–104, 2018.
Pearson, P. N. and Kučera, M.: Taxonomy, biostratigraphy, and phylogeny of Oligocene Turborotalita, in: Atlas of Oligocene planktonic foraminifera, edited by: Wade, B. S., Olsson, R. K., Pearson, P. N., Huber, B. T., and Berggren, W. A., Cushman Found. Foramin. Res. Sp. Publ., 46, 385–392, 2018.
Pearson, P. N., Shackleton, N. J., and Hall, M. A.: Stable isotopic evidence for the sympatric divergence of Globigerinoides trilobus and Orbulina universa (planktonic foraminifera), J. Geol. Soc. Lond., 154, 295–302, 1997.
Pearson, P. N., Olsson, R. K., Huber, B. T., Hemleben, C., and Berggren, W. A.: Atlas of Eocene planktonic foraminifera, Cushman Found. Foramin. Res. Sp. Publ., 41, 1–507, 2006.
Pearson, P. N., Wade, B. S., and Huber, B. T., Taxonomy, biostratigraphy, and phylogeny of Oligocene Globigerinitidae (Dipsidripella, Globigerinita and Tenuitella), in: Atlas of Oligocene planktonic foraminifera, edited by: Wade, B. S., Olsson, R. K., Pearson, P. N., Huber, B. T., and Berggren, W. A., Cushman Found. Foramin. Res. Sp. Publ., 46, 429–458, 2018.
Pearson, P. N. and Penny, L.: Coiling directions in the planktonic foraminifer Pulleniatina: A complex eco-evolutionary dynamic spanning millions of years, PLoS ONE, 16, p. 360, 2021.
Poole, C. R. and Wade, B. S.: Systematic taxonomy of the Trilobatus sacculifer plexus and descendant Globigerinoidesella fistulosa (planktonic foraminifera), J. Syst. Palaeontol., 17, 1989–2030, 2019.
Quillévéré, F., Morard, R., Escarguel, G., Douady, C. J., Ujiié, Y., de Garidel-Thoron, T., and de Vargas, C.: Global scale same-specimen morpho-genetic analysis of Truncorotalia truncatulinoides: a perspective on the morphological species concept in planktonic foraminifera, Palaeogeogr. Palaeoecol., 391, 2–12, 2013.
Rebotim, A., Voelker, A. H. L., Jonkers, L., Waniek, J. J., Meggers, H., Schiebel, R., Fraile, I., Schulz, M., and Kucera, M.: Factors controlling the depth habitat of planktonic foraminifera in the subtropical eastern North Atlantic, Biogeosciences, 14, 827–859, https://doi.org/10.5194/bg-14-827-2017, 2017.
Regenberg, M., Nielsen, S. N., Kuhnt, W., Holbourn, A., Garbe-Schönberg, D., and Andersen, N.: Morphological, geochemical, and ecological differences of the extant menardiform planktonic foraminifera Globorotalia menardii and Globorotalia cultrata, Mar. Micropaleontol., 74, 96–107, 2010,
Reuss, A. E.: Neue Foraminiferen aus den Schichten des Osterreichischen Tertiarbeckens, Denkschr. K. Akad. Wiss. Wien Math.-Naturwiss. Classe, 1, 365–390, 1850.
Rhumbler, L.: Nordische Plankton-Foraminiferen, in: Nordisches Plankton, edited by: Brandt, K., Lipsius und Tischer, Kiel Lief. 1, 14, 1–32, 1901.
Rhumbler, L.: Die Foraminiferen (Talamophoren) der Plankton-Expedition. Zugleich Entwurf eines natürlichen Systems der Foraminiferen auf Grund selektionistischer und mechanisch-physiologischer Faktoren. Ergebnisse der Plankton-Expedition der Humboldt-Stiftung, 3, 1–331, 1911.
Robbins, L. L.: Environmental significance of morphologic variability in open-ocean versus ocean-margin assemblages of Orbulina universa, J. Foramin. Res., 18, 326–333, 1988.
Rodhe, W.: Plankton, planktic, planktonic, Limnol. Oceanogr., 19, p. 360, 1974.
Rögl, F. and Bolli, H. M.: Holocene to Pleistocene planktonic foraminifera of Leg 15, Site 147 (Cariaco Basin Trench, Caribbean Sea) and their climatic interpretation, Initial Rep. Deep Sea, Leg 15, 553–615, 1973.
Rossignol, L., Eynaud, F., Bourget, J., Zaragosi, S., Fontanier, C., Elloz-Zimmermann, N., and Lanfumey, V.: High occurrence of Orbulina suturalis and “Praeorbulina-like specimens” in sediments of the northern Arabian Sea during the Last Glacial Maximum, Mar. Micropaleontol., 79, 100–113, 2011.
Saito, T. and Thompson, P. R.: The evolution and taxonomy of the foraminiferal genus Candeina d'Orbigny 1839, Abstracts with Programs, Geol. Soc. Am., 8, p. 1084, 1976.
Saito, T., Thompson, P. R., and Breger, D., Systematic index of Recent and Pleistocene planktonic foraminifera, University of Tokyo Press, Tokyo, 1–190, ISBN 0-86008-280-6, 1981.
Schiebel, R.: Planktic foraminiferal sedimentation and the marine calcite budget, Global Biogeochem. Cy., 16, 3-1–3-21, https://doi.org/10.1029/2001GB001459, 2002.
Schiebel, R. and Hemleben, C.: Planktic Foraminifers in the Modern Ocean, Springer, Berlin, Heidelberg, Springer, ISBN 978-3-662-50297-6, 2017.
Schwager, C.: Fossile Foraminiferen von Kar Nikobar, Reise der Österreichischen Fregatte Novara um Erde in den Jahren 1857, 1858, 1859 unten den Befehlen des Commodore B. Von Wuellerstorf-Urbair, Geologischer Theil (Zweite Abtheilung, Paläontologische Mittheilungen), 2, 187–268, 1866.
Setty, M. G. A. P.: Occurrence of Neogloboquadrina pachyderma new subspecies in the shelf-slope sediments of Northern Indian Ocean, Indian J. Mar. Sci., 6, 72–75, 1977.
Siccha, M. and Kucera, M.: ForCenS, a curated database of planktonic foraminifera census counts in marine surface sediment samples, Sci. Data, 4, 170109, https://doi.org/10.1038/sdata.2017.109, 2017.
Soldani, A.: Testaceographiae ac zoophytographiae parvae et microscopicae tomi primi pars altera in qua minuta et minima testacea ac zoophyte maris native vasculis inclusa aeneisquae tabulis insculpta describere et explicare pergit, Vol. 2, Sienna, Francisco Rossi, 1791.
Spezzaferri, S., Kucera, M., Pearson, P. N., Wade, B., Rappo, S., Poole, C., Morard, R., and Stalder, C.: Fossil and genetic evidence for the polyphyletic nature of the planktonic foraminifera “Globigerinoides”, and description of the new genus Trilobatus, PLoS ONE, 10, e0128108, https://doi.org/10.1371/journal.pone.0128108, 2015.
Spezzaferri, S., Coxall, H. K., Olsson, R. K., and Hemleben, C.: Taxonomy, biostratigraphy, and phylogeny of Oligocene Globigerina, Globigerinella, and Quiltyella, in: Atlas of Oligocene planktonic foraminifera, edited by: Wade, B. S., Olsson, R. K., Pearson, P. N., Huber, B. T., and Berggren, W. A., Cushman Found. Foramin. Res. Sp. Publ., 46, 179–214, 2018a.
Spezzaferri, S., Olsson, R. K., Hemleben, Ch., Wade, B. S., and Coxall, H. K.: Taxonomy, biostratigraphy, and phylogeny of Oligocene and lower Miocene Globoturborotalita, in: Atlas of Oligocene planktonic foraminifera, edited by: Wade, B. S., Olsson, R. K., Pearson, P. N., Huber, B. T., and Berggren, W. A., Cushman Found. Foramin. Res. Sp. Publ., 46, 231–268, 2018b.
Stainbank, S., Spezzaferri, S., Kroon, D., de Leau, E. S., and Rüggeberg, A.: The planktonic foraminifera Globigerinoides eoconglobatus n. sp. in a glacial–interglacial context: IODP359 Sites U1467 and U1468, Swiss J. Geosci., 111, 511–522, 2018.
Stainforth, R. M., Lamb, J. L., Luterbacher, H., Beard, J. H., and Jeffords, R. M.: Cenozoic planktonic foraminiferal zonation and characteristics of index forms, Univ. Kansas Paleontol. Contr., 62, 1–425, 1975.
Stainforth, R. M., Lamb, J. L. and Jeffords, R. M.: Rotalia menardii Parker, Jones & Brady, 1865 (Foraminiferida): Proposed suppression of lectotype and designation of neotype Z.N.(S.) 2145, Bull. Zool. Nomenclature, 34, 252–262, 1978.
Steineck, P. L. and Fleisher, R. L.: Towards the classical evolutionary reclassification of Cenozoic Globigerinacea (Foraminiferida), J. Paleontol., 52, 618–635, 1978.
Stuart, A.: Über Coscinosphaera ciliosa, eine neue Radiolarie, Zeitschr. Wiss. Zool., 16, 328–345, 1866.
Teichert, C.: Benthic – sí, planktic – no!, J. Paleontol., 55, 996–997, 1981.
Thompson, P. R.: Two New Late Pleistocene Planktonic Foraminifera from a Core in the Southwest Indian Ocean, Micropaleontology, 19, 469–474, https://doi.org/10.2307/1484909, 1973.
Todd, R.: Smaller foraminifera, in: Geology of Saipan, Mariana Islands, Pt. 3, Paleontology, U.S. Geological Survey, Professional Paper, U.S. Geological Survey, 280-H, 265–320, 1957.
Ujiié, Y. and Lipps, J. H.: Cryptic diversity in planktonic foraminifera in the northwest Pacific Ocean, J. Foramin. Res., 39, 145–154, 2009.
Ujiié, Y. and Ishitani, Y.: Evolution of a planktonic foraminifer during environmental changes in the tropical oceans, PLoS ONE, 11, e0148847, https://doi.org/10.1371/journal.pone.0148847, 2016.
Ujiié, Y., Kimoto, K., and Pawlowski, J.: Molecular evidence for an independent origin of modern triserial planktonic foraminifera from benthic ancestors, Mar. Micropaleontol., 69, 334–340, 2008.
Wade, B. S., Pearson, P. N., Berggren, W. A., and Pälike, H.: Review and revision of Cenozoic tropical planktonic foraminiferal biostratigraphy and calibration to the geomagnetic polarity and astronomical time scale, Earth-Sci. Rev., 104, 111–142, 2011.
Wade, B. S., Olsson, R. K., Pearson, P. N., Huber, B. T., and Berggren, W. A.: Atlas of Oligocene planktonic foraminifera, Cushman Foundation Special Publication, Cushman Foundation for Foraminiferal Research, ISBN electronic: 9781970168419 46, 524 pp., 2018a.
Wade, B. S., Pearson, P. N., Olsson, R. K., Fraass, A. J., Leckie, R. M., and Hemleben, C.: Taxonomy, biostratigraphy, and phylogeny of Oligocene and lower Miocene Dentoglobigerina and Globoquadrina, in: Atlas of Oligocene planktonic foraminifera, edited by: Wade, B. S., Olsson, R. K., Pearson, P. N., Huber, B. T., and Berggren, W. A., Cushman Found. Foramin. Res. Sp. Publ., 46, 331–384, 2018b.
Wang, L.: Isotopic signals in two morphotypes of Globigerinoides ruber (white) from the South China Sea: implications for monsoon climate change during the last glacial cycle, Palaeogeogr. Palaeoecol., 161, 381–394, 2000.
Weiner, A., Aurahs, R., Kurasawa, A., Kitazato, H., and Kucera, M.: Vertical niche partitioning between cryptic sibling species of a cosmopolitan marine planktonic protest, Mol. Ecol., 21, 4063–4073, 2012.
Weiner, A., Weinkauf, M., Kurasawa, A., Darling, K., Kucera, M., and Grimm, G. W.: Phylogeography of the tropical planktonic foraminifera lineage Globigerinella reveals isolation inconsistent with passive dispersal by ocean currents, PLoS ONE, 9, e92148, https://doi.org/10.1371/journal.pone.0092148, 2014.
Weiner, A., Weinkauf, M., Kurasawa, A., Darling, K. F., and Kucera, M.: Genetic and morphometric evidence for parallel evolution of the Globigerinella calida morphotype, Mar. Micropaleontol., 114, 19–35, 2015.
Wetzel, O.: Plate explanations of Rhumbler's “Plankton- Expedition”, The Micropaleontologist, 3, 33–40, 1949.
Wiesner, H.: Die Foraminiferen. In: Deutsche Südpolar Expedition 1901–1903, Bd. 20, edited by: von Drygalski, E., W. de Gruyter, W. de Gruyter & Co., Berlin, Leipzig, 53–165, 1931.
Williams, M., Schmidt, D. N., Wilkinson, I. P., Miller, C. G., and Taylor, P. D.: The type material of the Miocene to Recent species Globigerinoides sacculifer (Brady) revisited, J. Micropalaeontol., 25, 153–156, https://doi.org/10.1144/jm.25.2.153, 2006.
Williamson, W. C.: On the recent Foraminifera of Great Britain, The Ray Society, London, 1–107, https://doi.org/10.5962/bhl.title.139719, 1858.
WoRMS Editorial Board: World Register of Marine Species, https://doi.org/10.14284/170, http://www.marinespecies.org, last access: 4 April 2020.
Young, J. R., Wade, B. S., and Huber B. T. (Eds.): pforams@mikrotax website, http://www.mikrotax.org/pforams, last access: 4 April 2020.
- Abstract
- Introduction
- History of classification
- Revised classification
- Conclusions and outlook
- Annotated inventory of genera and species of extant planktonic foraminifera
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement
- Abstract
- Introduction
- History of classification
- Revised classification
- Conclusions and outlook
- Annotated inventory of genera and species of extant planktonic foraminifera
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement