the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Trace metal adsorption in three serial flow-through systems: implications for foraminiferal culturing experiments
Christian Helmut Gfatter
Jeremy D. Owens
Michael Martínez-Colón
Benthic foraminifera have been utilized extensively in laboratory culturing experiments to study the effects of elevated and/or depleted seawater concentrations of trace metals (e.g., on morphological shell abnormalities). In addition, past oceanic concentrations of various elements have been reconstructed through the analyses of benthic foraminiferal calcite. Paleoproxies of several trace metals have been used to track geologically ancient surficial processes (e.g., weathering and redox) by using black shales; however, analyses of foraminiferal calcite remain underconstrained for many transition metals. Growth experiments with trace elements, such as molybdenum (Mo) in this study, could be used to explore their potential as proxies to expand the sedimentary archives beyond those of black shales. Additional calcite proxies would provide more robust long-term paleo-redox reconstructions, especially for periods of Earth's history with limited archives. Concentrations of trace elements in seawater are often extremely low and measurements can be altered by adsorption, contamination, and/or stratification. To assess possible effects in calcifying organisms from trace metal concentration fluctuations, closed-system experiments using serial flow-through culturing devices can help ensure reliable results. Minor concentration changes in a trace element, particularly near or lower than seawater concentrations, can alter the equilibration time required for the modified source seawater to normalize with the seawater that has passed through the experimental setup. In this study, the stabilization was investigated after Mo-enriched seawater passed through three serial flow-through culturing systems. System A was an apparatus using a typical 24-well plastic cell culture plate, System B was based on System A but included modifications such as a customized block of polytetrafluoroethylene (PTFE) in place of the plastic cell culture plate, and System C was a serial system of synthetic perfluoroalkoxy alkane (PFA) vials connected using polytetrafluoroethylene tubing. The three systems were tested for the duration required to reach steady state to examine Mo adsorption by each system (A, B, and C) using 5-times and 2-times natural seawater concentrations of Mo ([Mo]sw). Though the three serial flow-through culturing systems reached equilibrium early in the process when [Mo]sw was 5-times seawater concentration, the experiments demonstrated that, when the Mo concentration was closer to modern seawater levels, systems reached equilibrium at different times. Specifically, systems that incorporated more adsorption-resistant materials and minimized seawater contact with “common” plastic (e.g., polypropylene and polystyrene) components reached equilibrium more quickly, where System C was the fastest. At low concentrations, equilibration takes longer due to the abundance of the element that can be adsorbed by components over time being lower, while the amount of time may become insignificant at far higher concentrations. Since equilibration time at lower concentrations with this trace metal differed by system, other foraminiferal trace element experiments should also consider the effect of adsorption. These setups were further tested in two experiments that compared the incorporation of Mo at various [Mo]sw into foraminiferal calcite, and their very low measurements, near current detection limits, demonstrate the importance of minimizing external effects such as adsorption. Importantly, with the best experimental setup tested, there was a small but measurable difference in the foraminiferal incorporation. Therefore, greater utilization of adsorption-resistant materials in experimental design is advised when studying very low oceanic elemental concentrations and their incorporation into foraminiferal calcite.
- Article
(6940 KB) - Full-text XML
- BibTeX
- EndNote
Paleoclimate studies have long since utilized foraminiferal carbonate tests to measure elemental concentrations that were incorporated during calcification using calibrations based on modern foraminiferal species (e.g., Katz et al., 2010; Emiliani, 1955; Shackleton, 1977; Chappell and Shackleton, 1986; Zachos et al., 1994; Miller et al., 2009). Additionally, minor elements of seawater, such as magnesium, have been studied (e.g., Lea, 1999; Lea et al., 2000; Lear et al., 2002, 2000). Moreover, analyses of trace metals, such as cadmium, zinc, barium, boron, uranium, and lithium, incorporated into foraminiferal calcite have created a suite of seawater proxies for paleoreconstructions (Katz et al., 2010; Lea, 1999). The trace element relationship between that found in foraminiferal calcite with respect to seawater is presumably expressed by an empirical partition coefficient (Morse and Bender, 1990), and many early determinations of this empirical partition coefficient were accomplished through calibration techniques using estimates of seawater chemistry and physical conditions at the collection site (e.g., Hester and Boyle, 1982; Lea and Boyle, 1989). However, all biological influences, such as temperature and light, on biogenic calcite composition are combined using this approach; consequently, initial laboratory studies culturing living planktic foraminifera were undertaken to determine more detail on biological effects (e.g., Delaney et al., 1985; Lea and Spero, 1992, 1994; Mashiotta et al., 1997; Lea et al., 1999). A comprehensive overview of planktic foraminifera that includes culture methods was written by Hemleben et al. (1989), which can be applied to any of the approximately 50 extant planktic foraminiferal species recognized (Hemleben et al., 1989; Schiebel and Hemleben, 2005), and new culturing methods continue to emerge for planktic foraminifera (e.g., Allen et al., 2016; Westgård et al., 2023; Meilland et al., 2024). However, thousands of benthic species exist, and laboratory studies using living benthic foraminifera also continue to develop (e.g., Chandler et al., 1996; Wilson-Finelli et al., 1998; Havach et al., 2001; Filipsson et al., 2010; Kramar et al., 2010; Barras et al., 2018; Diz et al., 2015; Smith et al., 2020). Importantly, certain benthic species are conducive to collect, maintain, and culture in a laboratory environment. Many controlled culturing experiments have been used to study foraminiferal responses like dormancy and propagation to environmental factors such as pH, temperature, and salinity (e.g., Bradshaw, 1957; Dissard et al., 2010; LeKieffre et al., 2017) and to study the effects of changes in elemental concentrations of foraminiferal tests. In one instance, a large 1600 L system was designed and implemented for culturing deep-sea benthic foraminifera, and the authors envisioned the apparatus as a way to conduct experiments to assess geochemical proxies (Hintz et al., 2004). However, smaller and less elaborate systems can be employed to study trace metal responses in the calcitic shells of shallow-water benthic foraminifera, such as Ammonia spp. and Elphidium spp. For example, heavy metal concentrations like copper (Cu) can be monitored, as they may reach toxic levels in a small body of water (e.g., bay or fjord) or potentially up to an entire basin due to anthropogenic pollution (e.g., Le Cadre and Debenay, 2006; Polovodova Asteman et al., 2015; Martínez-Colón et al., 2018; Schmidt et al., 2022).
Redox-sensitive trace metals such as molybdenum, uranium, vanadium, and zinc (Algeo and Maynard, 2004) – minor constituents in modern seawater including several transition metals – have been constrained for punctuated events (e.g., oceanic anoxic events) indirectly through black shale enrichments (e.g., Gill et al., 2011; Owens et al., 2016; Reinhard et al., 2013). Additionally, redox-sensitive trace elements affect enzymatic processes for primary producers (Bruland et al., 1991). In particular, while Mo is the most abundant transition metal in modern open-ocean oxic seawater conditions with concentrations of approximately 104 nM (e.g., Miller et al., 2011), where it is present in the form of molybdate (Algeo and Lyons, 2006; Kendall et al., 2017), low seawater Mo concentrations have been hypothesized to suggest significant increases in global anoxic/euxinic conditions during the geological past (e.g., Owens et al., 2016; Gill et al., 2011; Reinhard et al., 2013; Dickson et al., 2016). Even though benthic foraminifera have been used as local proxies in modern applications (e.g., as a bioindicator) and their usage in paleoceanography has mainly been associated with deep-ocean environments, research on redox-sensitive trace metals from foraminiferal calcite has been limited. Given their extensive distribution, benthic foraminifera have the potential to complement the local and global sedimentary oceanic archives. To test the utility of foraminifera to track the global seawater trace metal inventory using controlled culturing experiments, adsorption of Mo by three serial flow-through culturing systems was first assessed to ensure robust results. Data from subsequent experiments on extant benthic foraminifera were then utilized to evaluate the feasibility of using well-preserved foraminiferal tests as a proxy to reconstruct the marine trace metal concentration of redox-sensitive elements.
Laboratory experiments are time-consuming and require careful planning and preparation (Murray, 2006). Potential emerging proxies may come from trace metals that are normally in nanomolar (nM) concentrations in natural seawater, such as iodine at ≈ 450 nM, molybdenum at 104 nM, and vanadium at 35 nM (Bruland et al., 2014). However, bulk shell analyses of foraminiferal calcite concentrations by inductively coupled plasma mass spectrometry (ICP-MS) would be measured as parts per billion (ppb), since concentrations would not be those of a solute in solution. To study trace metal incorporation in the individual chambers formed during the experiment, the fluorescent dye calcein can be added to seawater utilized in growth experiments (Bernhard et al., 2004), and laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) analyses can be performed on specific test chambers (e.g., Filipsson et al., 2010; Barras et al., 2018). Consequently, advances in analytical precision in conjunction with very low seawater concentrations of trace elements and their effects on calcifying organisms require that thoughtful consideration be taken in experimental studies with living foraminifera. This study aims to quantify the time for the source input to reach equilibrium at 5-times and 2-times [Mo]sw for the three experimental systems that could provide a constant flow of the treated seawater to the benthic foraminifera in subsequent Mo-uptake research.
Three serial flow-through culturing systems (Fig. 1) were evaluated prior to two subsequent [Mo]sw experiments using benthic foraminifera in order to assess their performance with respect to minimizing fluctuations in elemental concentrations due to adsorption by the systems. The first experiment that assessed the uptake of Mo into foraminiferal calcite utilized seawater at 1×, 2×, and 3 × [Mo]sw, and a second experiment applied 1×, 3×, 5×, and 10 × [Mo]sw. All three systems had flat chamber bottoms but could have round or conically shaped chamber bottoms instead. Each system is described in the following sections.
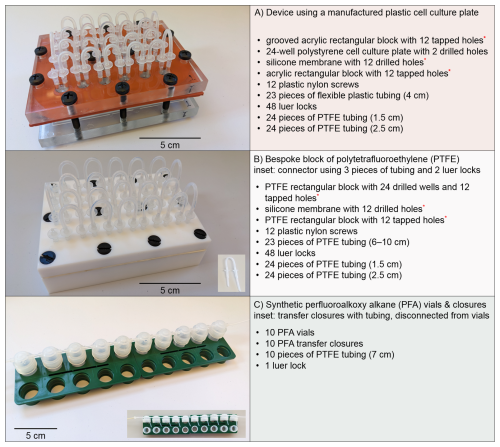
Figure 1Three serial flow-through culturing systems and components, excluding input and output tubing. System (A) is a polystyrene plastic cell culture plate between two acrylic plates, where chambers are coupled using silicone tubing. System (B) is a customized block of PTFE with a fitted PTFE lid, where wells are joined using PTFE tubing. System (C) is an interconnected series of synthetic perfluoroalkoxy alkane vials using PTFE tubing without luer locks. Custom components are indicated with a red asterisk (∗).
2.1 Serial flow-through culturing systems
Conceptually, serial flow-through culturing systems bring replenished, circumfluous seawater in contact with a single individual or a group of benthic foraminifera in each compartment, passing it through the first chamber to the next until the last. As long as stratification is avoided (Martínez-Colón, 2016), organisms are exposed to a flowing, consistent seawater treatment. For example, if a chamber's input and output were both near the top of the chamber, the source seawater – herein defined as the modified seawater used in an experiment (e.g., seawater with an increased [Cu]) – would remain in the upper layer, thereby reducing exposure to the foraminifera of the element(s) studied. Although the system is constantly being replenished with source seawater from the initial source seawater reservoir, there may be some seawater variability among system chambers due to foraminiferal uptake or other factors. A schematic diagram showing the hypothetical flow of seawater to and from a chamber is shown in Fig. 2. However, the components of the system itself may alter the consistency of the treatment (i.e., seawater with modified concentrations of one or more trace elements) due to adsorption by elements depending on their material. Therefore, before the start of an experiment utilizing foraminifera, the input of a system should be checked against its output until equilibrium has been reached.
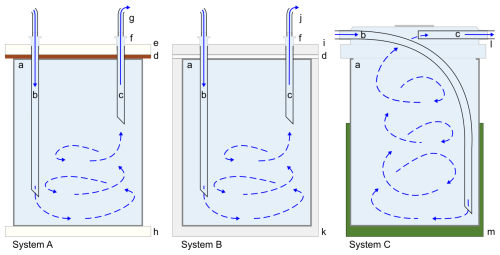
Figure 2Cross-sectional schematic diagram of seawater flow through a chamber of each system. Solid blue lines (“b” and “c” in each drawing) indicate known incoming and outgoing seawater flow, while blue arrows with dashed lines infer turbulent mixing based on tests with dyed water and not the actual direction of seawater flow in each system. Label descriptions: (a) chamber of a plastic well for System A and of a PTFE well for System B and interior of a PFA vial for System C, (b) PTFE tubing into the lower part of the chamber, (c) return PTFE tubing near the top of the chamber to circumvent stratification, (d) silicone gasket, (e) top acrylic plate, (f) plastic luer lock, (g) silicone tubing connecting the next chamber in the series, (h) bottom acrylic plate, (i) PTFE cover, (j) PTFE tubing connecting the next chamber in the series, (k) base PTFE plate, (l) PFA transfer closure, (m) plastic vial tray (optional). Illustration is not to scale.
Note that feeding benthic foraminifera during an experiment requires that the peristaltic pump be stopped so the chambers of a system can be accessed. Foraminifera can either be transferred to separate Petri dishes to be fed or be fed directly in place, which will reduce stress and the probability of harm. If an organism or group in each compartment is transferred to a Petri dish and fed, alterations or effects by food particles will be minimized, and each can then be returned to its location in the apparatus so the operation can be resumed. Additionally, external feeding using sterile Petri dishes may reduce the risk of contamination to the entire system. If foraminifera are directly fed in the chambers of a system instead, handling specimens will be minimized and the process will be more straightforward, but residual food particles and/or additional metabolites may affect outcomes. However, for the experiments on the systems, foraminifera were not used, as the focus was on adsorption by the plastic components.
2.1.1 System A: polystyrene plastic cell culture plate
The first vessel (Fig. 1a) was a modified version of the one originally designed and utilized to analyze [Cu] in foraminiferal calcite after exposure to high levels of seawater concentrations (de Nooijer et al., 2007). Martínez-Colón (2016) tested the system by running colored distilled water through the vessel and observed stratification in the chambers. This problem was solved by introducing a longer “inflow” piece of tubing that extended to near the bottom of the chamber and a shorter “outflow” piece of tubing. For these trials, a single device consisting of a 24-well polystyrene plastic cell culture plate was inserted between two acrylic plates. Acrylic or polymethyl methacrylate (PMMA), more commonly known by various trade names such as Plexiglas® and Lucite®, is a lightweight and shatter-resistant transparent plastic compared to glass. A silicone membrane with circular holes corresponding to the wells was placed above the cell culture plate to create a seal between it and the upper acrylic plate, and the unit was secured by 12 plastic nylon screws. The upper and lower plates were machined with holes for the screws and positioned to balance compression of the plates. Individual 3.5 mL compartments or wells were connected by short pieces of silicone tubing, roughly 4 cm in length, using luer locks (i.e., straight polypropylene fittings with a male luer slip to a hose barb adapter). Each well had a longer piece of polytetrafluoroethylene (PTFE) tubing (≈ 2.5 cm) connected to a luer lock for incoming seawater and shorter one (≈ 1.5 cm) connected to the other luer lock for seawater flowing to the next well or final collection container. Seawater mixing was verified by testing the system with colored distilled water. The first well in the series was connected to the PharMed® biocompatible pump tubing (BPT), composed of a polypropylene-based thermoplastic elastomer or rubber, used by the peristaltic pump. The last well was connected to PTFE tubing leading to the collection jar using a custom-made connector, 0.5 cm of BPT attached to a luer lock. All 24 wells were used for a single treatment in the configuration of the apparatus.
2.1.2 System B: customized block of polytetrafluoroethylene (PTFE)
The next device (Fig. 1b) was a reconceptualized adaptation based on the aforementioned vessel using a bespoke block of PTFE and was also utilized to analyze [Cu] in foraminiferal calcite (Martínez-Colón, 2016). Both PTFE components were machined as specified by the details within the aforesaid article. PTFE, also referred to by the trade name, Teflon®, is hydrophobic and has a very low coefficient of friction. The device consisted of a PTFE base with 24 wells, a comparable silicone membrane as previously described placed above the base, and a PTFE lid. The unit was also secured by 12 plastic nylon screws, but the arrangement was altered from the preceding design using a cell culture plate to evenly compress the apparatus, as PTFE is softer than acrylic. Separate 4 mL compartments were connected by short pieces of PTFE tubing, 6–10 cm in length, using luer locks. Because PTFE is not as flexible as silicone, longer pieces were used to eliminate kinks that alter the diameter of the tubing, thereby impeding flow. For these trials, heat was applied during the shaping of the PTFE pieces to achieve arcs with shorter lengths, whereas a previous configuration used longer PTFE pieces to avoid kinks. As mentioned in Sect. 2.1.1, each well had a longer and shorter piece of PTFE tubing connected to the luer locks to ensure mixing of the seawater. Also, as before, the initial luer lock of first well in the series was connected to the BPT used by the pump, and the last was linked to a collection jar by a custom-made connector and PTFE tubing. All wells of the PTFE unit were used for a single treatment in the setup. In addition, the unit was tested beforehand using dyed distilled water to ensure that seawater would not stratify and flow serially through all wells. The surface of the PTFE blocks can deform slightly over time due to its relative softness; therefore, despite the silicone membrane, uneven areas can develop that allow the seawater flow to circumvent its intended route. The unit utilized in these experiments did not have such an issue; thus the two blocks did not have to be planed to re-establish uniform thickness and level surfaces; however, planing the blocks periodically to keep the block flat across the top is recommended to maintain a leak-free system.
2.1.3 System C: synthetic perfluoroalkoxy alkane (PFA) vials
The third experimental setup (Fig. 1c) was a serial system of perfluoroalkoxy alkane (PFA) vials and transfer closures connected by PTFE tubing, re-engineered from the prior two culturing designs in order to support redox-sensitive trace metal research using controlled culturing experiments (Gfatter and Owens, 2021). The system was not limited by number of vials, but, rather than using 24 vials to create 24 chambers like the previous devices, 10 PFA vials were selected, as the 7 mL volume of a vial was greater than that of a well in either previously discussed system. A total of ten 7 cm pieces of PTFE tubing (i.e., a PTFE “connector”), with one side cut at a 45° angle, connected the PFA transfer closures, each with two openings. For every transfer closure, one opening was fitted with a piece of tubing where the angled side of the PTFE connector would reach the bottom of the vial when the transfer closure caps the vial. Because of the tight tolerance of the opening and the limited flexibility of the tubing, a new strong rubber band was used to help the PTFE connector make the bend beyond the inside rim (Fig. 3). After the PTFE connector with the angled end was inserted to the center of the closure, the rubber band was placed so that it could be pulled downward while the connector was pushed forward, thereby forcing the connector down and away from the opening on the opposite side. Rubber bands utilized in the technique were discarded after all PTFE connectors were completed.
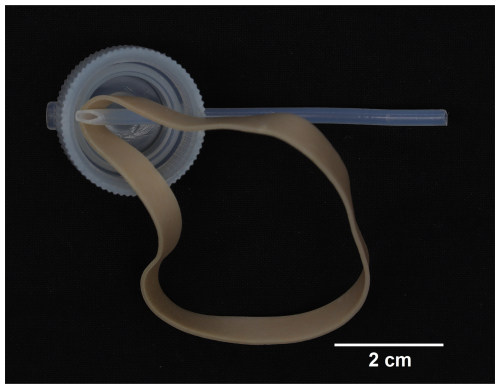
Figure 3Underside view of the PTFE connector inserted into the PFA transfer closure where a new rubber band, positioned as demonstrated, was then pulled taut to assist with bending the piece into place as shown in Fig. 4.

Figure 4PFA transfer closures connected in sequence for the last 3 of 10 flat bottom PFA vials as shown in Fig. 5.
Aside from the first vial, the external portion of the connector by the transfer opening was placed into the opening of the preceding transfer closure (Fig. 4). Once the 10 transfer closures were interconnected, each vial was positioned to receive a connector and then screwed tightly into its transfer closure. The connector of the first transfer closure was coupled with a luer lock to the BPT used by the pump, while the last transfer closure was connected directly to PTFE tubing leading to the collection jar (Fig. 5). Seawater flowed into the bottom of the vial with this configuration, where the angled side prevented a restriction of flow; thus no stratification occurred out of the top as well. The setup was tested to ensure there was no stratification: the flow of the system was checked with colored distilled water, thus verifying that the solution was mixed. Two plastic vial trays were used to organize the series of vials, though not essential for the system.
The use of manufactured PFA vials and closures, typically utilized in trace metal research, along with the PTFE tubing, minimizes elemental adsorption, which is required for low-concentration experiments. The serial connection ensures a uniform flow of the seawater through all vials.
2.2 Seawater preparation and experimental setup
Source seawater was collected into a cleaned and rinsed 50 L Nalgene™ low-density polyethylene (LDPE) carboy from the Florida State University (FSU) Coastal and Marine Lab in St. Teresa, FL, where seawater from the Gulf of Mexico is drawn into holding tanks through pipes, sand-filtered, and then passed through a cartridge filtration system using 10 µm filters. At FSU in Tallahassee, the seawater was additionally filtered to 0.2 µm, using polycarbonate and nylon membrane filters, and stored in a 20 L Nalgene™ LDPE carboy. The seawater in the carboy had a pH of 8.14 at 21.9 °C, and salinity was 29.5 ‰. The initial and filtered seawater [Mo] from the Gulf of Mexico was 102 and 120 nM, respectively. All experiments were conducted at a temperature of 20–22 °C and exposed to indirect sunlight for approximately 12 h. A total of 4 L of the 0.2 µm filtered natural seawater was placed in a high-density polyethylene (HDPE) container. The concentration of Mo in natural seawater is 100–108 nM (e.g., Bruland et al., 2014; Collier, 1985; Morford and Emerson, 1999; Miller et al., 2011), and Mo from a stock solution by Inorganic Ventures was added so that the concentration in the container would be about 5 times that of natural seawater. The experiment with a lower [Mo] used the same HDPE container after being cleaned, but Mo was added to 4 L of 0.2 µm filtered seawater to reach approximately twice that of the Mo concentration in natural seawater.
For both experiments, three new 95 cm pieces of PTFE tubing – to bring source seawater from the reservoir into each of the three systems – were placed into holes made in the cap, reaching the bottom of the container, and then connected to a 406 mm 3-stop PharMed® BPT (0.89 mm tubing inner diameter) with a luer lock. Flexible tubing was needed for the peristaltic pump, and this tubing made from a polypropylene-based thermoplastic elastomer, which has broad chemical resistance, outlasts silicone tubing. The first and second stops (i.e., plastic tabs on the tubing) of each BPT piece were fitted into a cartridge for the channels of an Ismatec® C.P. 78017-10 peristaltic pump and then connected to a luer lock that fed into one of the three serial flow-through systems (Fig. 6). The first two stops were utilized, so the outgoing portion of tubing was longer and thus connected to the systems without difficulty. The peristaltic pump was set to advance seawater from the reservoir at 9 mL h−1 through each system, as previously done by de Nooijer et al. (2007) and Martínez-Colón (2016). This rate provided ample mixing as observed in the tests of each system for stratification using dyed distilled water. A rate of 9 mL h−1 allowed ≈ 2–3 exchanges of seawater in each system chamber for System A and System B but only ≈ 1.5 for System C, which would need a separate peristaltic pump to reach a similar exchange. At each collection interval, source seawater samples of ≈3 mL were pipetted from the reservoir. Next, system samples of ≈3 mL were taken directly from the output tubing into test tubes for approximately 20 min. An initial sample of the untreated natural seawater was taken along with periodic samples from the experiments. During each trial, excess seawater between collection times was collected in amber Nalgene™ HDPE bottles.
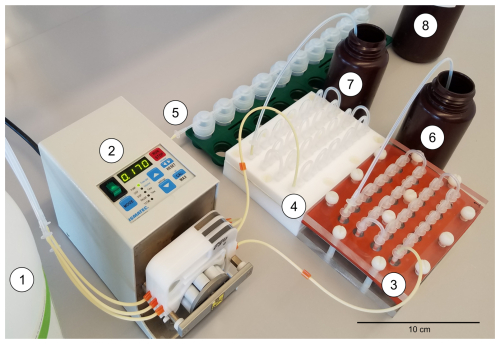
Figure 6Experimental setup of components and culturing systems where seawater from the reservoir (1) was drawn by the peristaltic pump (2) and advanced through each of the three serial flow-through systems (3, 4, and 5) and then into the output collection bottles (6, 7, and 8), which were capped between sampling periods.
2.3 Collection of algae and juvenile benthic foraminifera
Sediment samples were collected from the Wadden Sea mudflat in Den Oever, NL (52°56′ 25.2′′ N, 5°01′ 24.9′′ E), and later stored in an environmental chamber set at 10 °C. The sediments were then sieved to 75–90 µm and stored in the environmental chamber. Prior to the start of an experiment, portions of 75–90 µm sediment were placed in Petri dishes, and a 3–5 mL aliquot of the chlorophyte, D. perfecta, was added. After 24 h, living foraminifera – identified by the uptake of the green algae causing a color change in the foraminifera – were picked from the other particles and sorted by genera (Ammonia spp., Elphidium spp., or other spp.). The primary species sought was Ammonia cf. molecular type T6 or T7 (North Sea tidal flats and US Atlantic coastal microtidal marshes, respectively; Hayward et al., 2004), herein referred to as Ammonia tepida, which is readily found in mud flats and coastal areas. Although Richirt et al. (2019) found that species may be distinguished by their morphological characteristics, A. tepida was not sorted further. Lots of A. tepida (100 specimens) were utilized for each treatment in an experiment.
Six cultures of algae (two Chlamydomonas spp., Dunaliella tertiolecta, Dunaliella salina, Phaeodactylum tricornutum, and Isochrysis galbana) used in previous foraminiferal growth studies with Ammonia spp. (e.g., Bernhard et al., 2004; Bradshaw, 1957; de Nooijer et al., 2007; Munsel et al., 2010; van Dijk et al., 2019), along with three additional cultures of algae (Porphyridium cruentum, Tisochrysis lutea, and Dunaliella primolecta), were obtained from the Milford Laboratory of the National Oceanic and Atmospheric Administration. The cultures were maintained independently using f/2 medium, an enriched seawater medium made with nutrients for marine algae and artificial seawater, and split every 2–3 weeks with added f/2 medium. Algal subsamples of the nine different algal cultures were taken monthly, combined into a container, and then killed via heat to be used later as foraminiferal food.
2.4 Experimental setup to test molybdenum incorporated into foraminiferal calcite
System C was utilized for both experiments utilizing A. tepida. For the first Mo uptake experiment, filtered seawater was prepared in the same manner as described in Section 2.2. After filtration, the seawater had a pH of 8.17 at 21.0 °C, and salinity was 30.7 ‰. The salinity of the Wadden Sea mudflats ranges between approximately 20 ‰ and 30 ‰ and salinity of the filtered seawater was within the range needed for normal growth and reproduction by A. tepida (Bradshaw, 1957). Seawater treatments with elevated concentrations of Mo were made with stock liquid Mo from Inorganic Ventures to create 2× and 3 × [Mo]sw, while untreated seawater represented the control of 1 × [Mo]sw. Seawater treatments of 3×, 5×, and 10 × [Mo]sw also took place for the second Mo uptake experiment.
For each seawater treatment and control, approximately 100 juvenile specimens of A. tepida, ≈ 63–90 µm in diameter, were placed in 10 PFA vials with 0.5 mL of algal detritus. A single feeding at the start of each experiment was chosen for simplicity to replicate. After 8 weeks, the experiment was stopped, and foraminiferal tests greater than 90 µm were collected. A portion of the original samples, about 25–45 individuals depending on the shell size, was transferred into 4 mL PFA vials to be analyzed for elemental concentrations via ICP-MS. All shells in each of the foraminiferal calcite samples were first dissolved in 80 µL of twice-distilled trace-metal-grade nitric acid (HNO3) for 24–48 h. For analysis, the samples were then diluted to 2 % HNO3 using ultrapure (i.e., Type 1) water.
2.5 Data collection and analyses
Seawater molybdenum analyses were performed utilizing an Agilent 7500cs quadrupole inductively coupled plasma mass spectrometer (ICP-MS) using standard procedures as outlined in previous work from the Geochemistry Group at the National High Magnetic Field Laboratory (NHMFL) at FSU (e.g., Lowery et al., 2018; Bowman et al., 2020; Kozik et al., 2022; Them et al., 2022). Calibration standards were attained by serial dilution of a nitric acid solution composed of 21 High-Purity Standards or Inorganic Ventures ICP-MS standard elements. The calibration standards for seawater samples were not matrix-matched because all salts contain trace metals, which would introduce errors in the measurements. Excluding the blank, Mo had a range of 0–2500 nM in these standards. Aliquots of the seawater samples were diluted 10 : 1 with trace-metal-grade 2 % HNO3 and then analyzed with the Agilent 7500cs ICP-MS. The precision of the instrument, measured by relative standard deviation of Mo, for the 5 × [Mo]sw analysis was approximately 4.6 % and about 1.5 % for the 2 × [Mo]sw analysis. The average counts per second were roughly 49 000 and 24 000, respectively. The conversion of the quantitative output of Mo to nM was verified with the calculation of calcium (Ca) from its measurements, which remained nearly constant, as it should not have changed given the source seawater was the same for all Mo concentration samples.
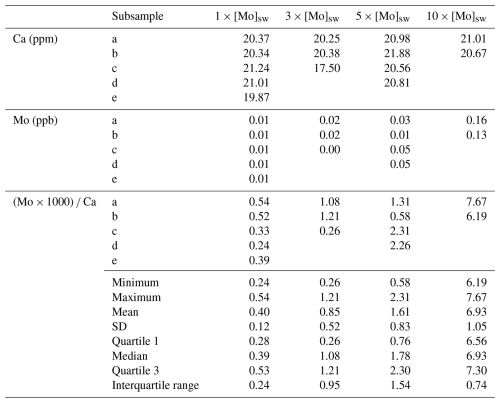
Foraminiferal calcite molybdenum analyses were performed on a Thermo Fisher Scientific Element 2 ICP-MS using the standard procedures as noted above at the NHMFL. Measurements of Mo (ppb) and Ca (ppm) were collected, and the calibration standards for foraminiferal samples were matrix-matched to Ca at 20 ± 5 ppm. That is, matrix effects that interfere with or attenuate the measurements due to variable amounts of Ca were minimized by matching samples within 25 % of the standards for Ca values. Samples that did not match the Ca target were rediluted with 2 % HNO3 and reanalyzed. Molybdenum–calcium ratios () are presented as Mo (× 1000) ppm Ca ppm to normalize for the amount of foraminiferal calcite dissolved, which is similar to other elemental applications (e.g., Lu et al., 2010; McCorkle et al., 1995; Rosenthal et al., 1997; Lea et al., 1999; Hintz et al., 2006a, b).
The three serial flow-through culturing systems were assessed on their ability to reach equilibrium quickly using a higher and lower [Mo]sw. This information was reviewed to select the system for the subsequent experiments using foraminifera. Seawater concentrations of Mo at 1×, 2×, and 3 × [Mo]sw were used in the first experiment that assessed the uptake of Mo into foraminiferal calcite, and seawater concentrations of Mo at 1×, 3×, 5×, and 10 × [Mo]sw were utilized in the next. Results are presented in the following subsections.
3.1 Equilibration time of the serial flow-through culturing systems
A seawater standard solution – used to compare seawater elemental concentrations to those measured from actual seawater samples – was measured and calculated to be 105 nM for Mo, whereas the initial seawater and filtered seawater from the Gulf of Mexico were 102 and 120 nM for Mo, respectively. The source seawater in the trial that tested the devices at 5-times [Mo]sw had a mean of 564 nM (Table A1 in the Appendix) and was 579 nM at the onset of the trial, and all three configurations had an average value within 5 nM of 564 nM. The source seawater at the beginning of the experiment measured fractionally higher than 578 nM, but this measurement was less than 1 nM beyond the 95 % confidence interval (i.e., within 2 standard deviations of the mean) of 551–578 nM, and data remained within that range thereafter. Furthermore, the means of all three systems were within 2 standard deviations of the mean source seawater value.
The [Mo]sw measurements at the beginning of this trial were 557, 559, and 562 nM for System A, System B, and System C, respectively (Fig. 7). Samples were collected for the seawater and all systems at the onset, 6 h point, 12 h point, and then every 24 h starting from the 24 h point forward. This trial ran for 7 d, and the 40 samples were subsequently analyzed for [Mo]. At the 168 h mark, all four measurements were 560 ± 3 nM. Although there may be some variability prior to the 6 h point, all serial flow-through systems reached equilibrium by the 6 h mark.
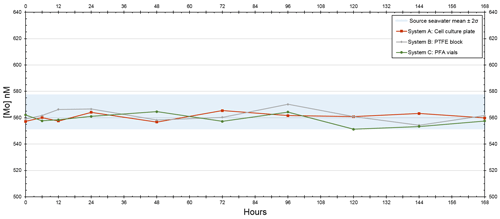
Figure 7Molybdenum concentrations of seawater after being moved by a peristaltic pump through each culturing system, where the source was 5 times that of natural seawater. The blue bar highlights the 95 % confidence interval of the source seawater (mean ± 2 standard deviations), where the mean was 564 nM and the range was 551–578 nM.
For the trial that tested the devices at twice [Mo]sw, source seawater had an average value of 287 nM (Table A2), and all initial measurements were within the 95 % confidence interval of 268–306 nM. The experiment at the lower [Mo] had more variability between the systems. The standard deviation of System A (24 nM) was nearly 3 times higher than that of the source seawater (9 nM), whereas System B (18 nM) was about twice as high. On the other hand, the standard deviation of System C (4 nM) was much lower than that of System A and System B and lower than that of the source seawater.
The [Mo]sw measurements after the source seawater flowed through the devices for 1 h were 293, 280, and 284 nM for System A, System B, and System C, respectively (Fig. 8). Samples were collected for the seawater and all systems at the 1 h point and then every 12 h from the 12 h point forward. This trial ran for 5 d, and the 44 samples were subsequently analyzed for [Mo]. The first measurement of the source seawater was 299 nM and, aside from one measurement less than 1 nM below 268 nM recorded at the 60 h mark, remained within the 95 % confidence interval. System A trended downward, falling below the lowest seawater measurement of 268 nM about halfway through the experiment, until the 108 h mark. System B had two values that diverged beyond the 268–299 nM range of the source seawater at the 12 and 48 h points and then remained within the 95 % confidence interval except for the measurement of 322 nM at the 108 h point. The measurements from System C ranged from 272 to 287 nM, with an average value of 281 nM – comparable to that of 287 nM for the source seawater. Although only System C equilibrated with the source seawater from the start, measurements of all serial flow-through systems returned to the 95 % confidence interval by the fifth day.
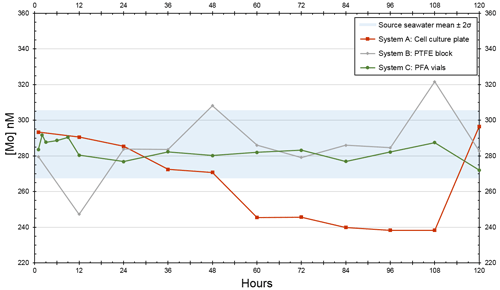
Figure 8Molybdenum concentrations of seawater after being moved by a peristaltic pump through each culturing system, where the source was twice that of natural seawater. The blue bar highlights the 95 % confidence interval of the source seawater (mean ± 2 standard deviations), where the mean was 287 nM and the range was 268–306 nM.
Since System C remained within the 95 % confidence interval for all periods, additional measurements were taken at 2, 3, 6, and 9 h intervals. These four measurements (292, 288, 289, and 290 nM, respectively) were between 288 and 292 nM. Although slightly higher than its 272–287 nM range, all measurements still remained within the 95 % confidence interval of the source seawater. These additional measurements suggest that System C reached equilibrium with the 2 × [Mo]sw source seawater in no more than 2 h. Thus, it is likely that System C reached equilibrium with the 5 × [Mo]sw source seawater by the 2 h point as well.
3.2 Molybdenum incorporation into foraminiferal calcite
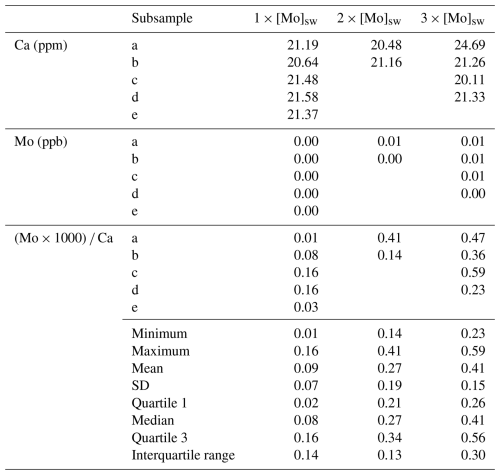
The seawater Mo concentration measurements obtained from the Agilent 7500cs ICP-MS for the 1×, 2×, 3×, 5×, and 10 × [Mo]sw used in the foraminiferal uptake experiments were 97, 209, 329, 554, and 1025 nM, respectively. The first experiment compared foraminiferal uptake of Mo at concentrations of 1×, 2×, and 3 × [Mo]sw, and the average ratios were 0.09, 0.27, and 0.41 with standard deviations of 0.07, 0.19, and 0.15, respectively (Fig. 9). The 2 × [Mo]sw only had two values (Table A3); thus the statistical importance of these data is less robust. However, values from 1× and 3 × [Mo]sw had some separation, as indicated by the error bars (mean ± 1 standard deviation) and the box and whisker plots (Fig. 9). Ratios from the first set of foraminiferal calcite samples had a narrow spread, ranging from a minimum of 0.01 for 1 × [Mo]sw to a maximum of 0.59 for 3 × [Mo]sw.
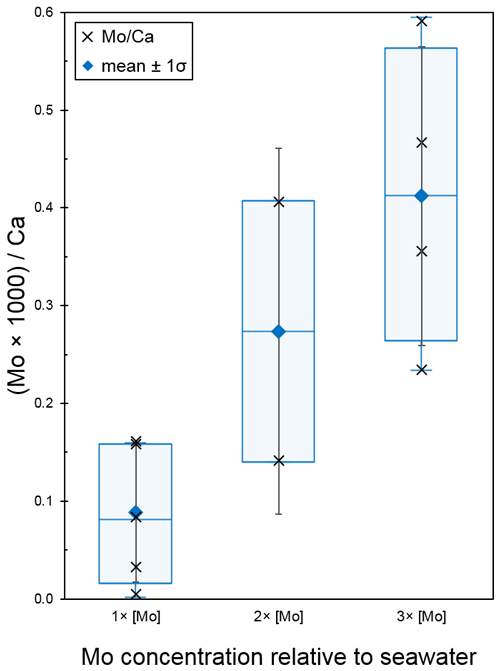
Figure 9Incorporation of Mo into calcite by A. tepida at 1, 2, and 3 times the concentration of seawater. The data are represented by a cross (i.e., “×”). For each concentration, the blue diamond shows the average , with black error bars indicating ±1 standard deviation. In addition, the blue center line of the boxplot represents the median, the blue box shows the interquartile range, and the blue whiskers mark the minimum and maximum values.
The second Mo uptake experiment tested seawater concentrations of Mo at 1×, 3×, 5×, and 10 × [Mo]sw, and the average ratios were 0.40, 0.85, 1.61, and 6.93 with standard deviations of 0.12, 0.52, 0.83, and 1.05, respectively (Fig. 10). Two possible outliers of 0.26 for 3 × [Mo]sw and 0.58 for 5 × [Mo]sw (Table A4) may have distorted the results. Exclusion of those data would have shifted the error bars and altered the box and whisker plot such that there would have been no overlap between all four [Mo]sw compositions; therefore, the differences between 1×, 5×, and 10 × [Mo]sw may be statistically significant, but further work is needed. Ratios from the second set of foraminiferal calcite samples had a wider spread, ranging from a minimum of 0.24 for 1 × [Mo]sw to a maximum of 7.67 for 10 × [Mo]sw.
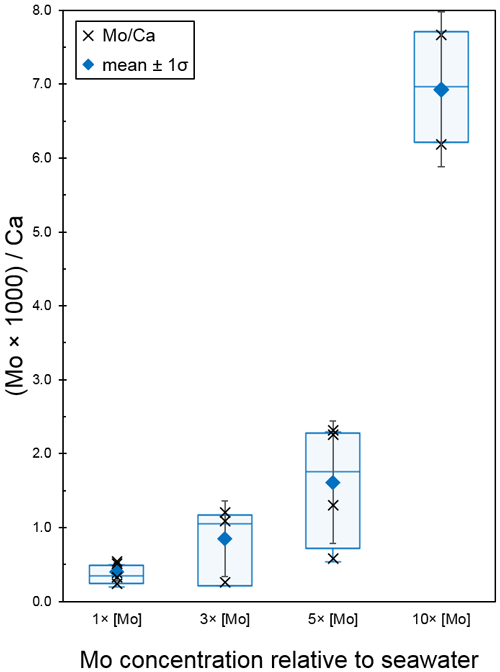
Figure 10Incorporation of Mo into calcite by A. tepida at 1, 3, 5, and 10 times the concentration of seawater. The data are represented by a cross (i.e., “×”). For each concentration, the blue diamond shows the average , with black error bars indicating ±1 standard deviation. In addition, the blue center line of the boxplot represents the median, the blue box shows the interquartile range, and the blue whiskers mark the minimum and maximum values.
All three serial flow-through systems stabilized within 6 h using the higher [Mo]sw, but System C was the fastest to reach equilibrium using the lower [Mo]sw. Since System C reached equilibrium with the 2 × [Mo]sw within 2 h, it is likely that System C also reached equilibrium that quickly with the 5 × [Mo]sw. Because of its performance and consistency with both the lower and higher [Mo]sw, System C was chosen for our Mo-uptake experiments and considered more suitable for experiments with low and high trace metal concentrations. The impact of metabolite accumulation is also likely interrelated to the number of system chambers and quantity of foraminifera utilized in a serial system, so configuration flexibility contributed to our decision to use System C.
4.1 Evaluation of the three serial flow-through culturing systems
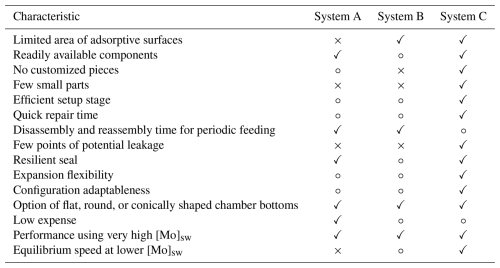
System A utilizing the plastic cell culture plate was comparatively inexpensive to construct and has many readily available parts, although the acrylic plates and silicone sheet required additional machining. It was easier to disassemble and reassemble if feeding and/or examining organisms during trials was needed; therefore, it was practical for experiments that isolated individual benthic foraminifera or groups. Because of the many adsorptive surfaces, System A did not perform well at the lower [Mo]sw with an average of 287 nM. Specifically, [Mo] declined from the onset and dropped below the 95 % confidence interval of 268–306 nM after the 48 h mark. Furthermore, [Mo] did not return to the 268–306 nM range until the final analysis. Despite improving to 296 nM, which was within the 95 % confidence interval, the high standard deviation of 24 nM insinuates that more time may be needed to determine if equilibrium is maintained by System A when utilized in experiments with low [Mo]. This rapid return to the range considered in equilibrium with the source seawater may be a result of its surfaces becoming saturated with Mo. During this time between 108 and 120 h, it is possible that the system became supersaturated, which could release Mo from the chamber walls to the overlying seawater. Additional data from shorter intervals and from periods beyond 120 h would help to identify the mechanism driving the observed changes in System A (Fig. 8). System A, though, did perform well at the higher [Mo]sw with an average of 564 nM. Here, notwithstanding minor fluctuations, it maintained measurements within the 95 % confidence interval of 551–578 nM (Fig. 7). As a result, it is likely that this system is unsuitable for experiments with lower trace metal concentration, but the configuration may be useful and cost-effective for trials utilizing high elemental concentrations. Three similar devices (i.e., without the adaptation to circumvent stratification) were used by de Nooijer et al. (2007), who studied six treatments, and each device served two treatments employing 12 wells of the plastic cell culture plate. Four foraminiferal specimens per well were used in their first experiment, and only two specimens of another species per well were used in the second. The range of Cu in natural seawater is 0.4–5 nM, with an average concentration of ≈3 nM (Bruland et al., 2014), whereas de Nooijer et al. (2007) used enrichments of 0, 0.10, 0.20, 0.50, 10, and 20 µM. The initial enrichment of 100 nM was about 30 × [Cu]sw, so the experimental concentrations were exceptionally high compared to natural seawater and thus likely brought the system to equilibrium quickly. However, System A has over 100 small parts and requires a few hours to set up. The repair time is modest, for example, if a luer lock were to break during operation, but there are many points of potential leakage.
System B, implementing the bespoke PTFE block with machined wells, offers a solution for experiments with lower elemental concentration that benefit by having fewer adsorptive surfaces, and it is well adapted to experiments on individual benthic foraminifera or groups that require routine feeding (e.g., twice weekly). However, it was more expensive to produce and required considerable customization. Because of fewer adsorptive surfaces, it performed well at the lower [Mo]sw, only deviating three times from the 95 % confidence interval of 268–306 nM, with values of 247, 308, and 322 nM (Fig. 8). Although the 322 nM was the penultimate measurement, which then returned back within the 95 % confidence interval, System B had four prior measurements that were in the range; hence, 5 d is considered the probable time needed for the system to reach equilibrium for lower trace metal concentrations. System B also performed well at the higher [Mo]sw, where small oscillations were well within the 95 % confidence interval. Accordingly, this system is considered appropriate for experiments with a wide range of trace metal concentrations, but equilibration time needed for a specific element should be determined beforehand. Its functionality is comparable to System A, which employs a plastic cell culture plate. Similarly, it has more than 100 small parts, a lengthy setup time, and numerous points of possible failure. Maintenance time varies, for example, if a luer lock versus a nylon screw were to break. Furthermore, a unit must be checked periodically as the seal weakens over time; that is, flow to all cells should be verified routinely. While de Nooijer et al. (2007) used high enrichments of Cu in their study, Martínez-Colón (2016) applied lower enrichments of 0, 0.22, and 0.32 µM and thus sought to minimize Cu adsorption by the system itself through the application of trace-metal-resistant materials where possible. For those experiments, only one foraminiferal specimen was placed in each well. Martínez-Colón (2016) determined that, with a lower Cu enrichment of approximately 0.38 µM, the unit reached equilibrium at the fifth day of a 7 d trial. At the lower [Mo], the unit may have reached equilibrium after 60 h (2 d) if the anomalous measurement at the 108 h point was rejected; otherwise, 5 d would be the minimum required for lower [Mo] to reach equilibrium throughout the system, similar to lower [Cu]. Conversely, System B required considerably less time to reach equilibrium for higher [Mo], likely within 6 h, and comparable performance is hypothesized with higher concentrations of other trace elements, as adsorption by non-PTFE components should be faster due to greater exposure, while adsorption-resistant surface areas repel most elements.
System C, composed of synthetic PFA vials, was configured with readily available parts with the least adsorptive surfaces. An assortment of vial volumes is available that provides adaptability to monitor trace metal effects on larger organisms or groups, and, although the configuration herein used 10 vials, the number of vials utilized is at the discretion of the researchers. Due to the minimal amount of adsorptive surfaces, it performed well at the lower [Mo]sw, always within the 95 % confidence interval. As expected, it performed well at the higher [Mo]sw, also never deviating from the 95 % confidence interval. Moreover, in both the low- and high-[Mo] experiments, equilibrium was reached quickly, likely 2 h or sooner. In addition, the standard deviation in both experiments was about 4–5 nM. As a result of its performance, System C is considered suitable for experiments with low or high trace metal concentration. After the PTFE connectors are in place, which can take 5–15 min per PFA transfer closure, the setup time is quick, requiring approximately 15 min to configure a 10-vial system. Notably, a PTFE connector and associated PFA transfer closure can be kept together when it is cleaned for future use. Since compartments are unitary and there are no customized parts, maintenance is straightforward. For example, it is possible for a transfer closure to have one of its openings lose its tight tolerance, thereby causing a leak, and would thus be replaced immediately with another transfer closure. Even so, the PTFE tubing fits very tightly in the transfer closure openings, and there are fewer points of leakage in a 10-vial system. Because the vials themselves need to be turned in order to disassemble and reassemble the system, the setup is more practical for trials on groups of organisms such as 5–10 foraminifera. The main caveat of this system is the expense of the PFA vials and transfer closures, but those components are durable and reusable. For reuse, items were first soaked in ultrapure (i.e., Type 1) water, the PFA container was sealed and agitated, and then excess water was emptied. Next, 2 % high-purity nitric acid was added to the container with the components, and then the sealed container was placed on a hot plate at 100 °C for a minimum of 24 h. These steps were repeated once more followed by a final rinse using ultrapure water. Other components, such as the reservoir and bottles, were rinsed three times with ultrapure water, soaked in a nitric acid bath, and then triple-rinsed with ultrapure water. The configuration of System C used 10 PFA vials and transfer closures, and all components cost slightly more than those in System B; however, larger or more vials and transfer closures could appreciably increase initial setup expenditures. Nevertheless, the highest expense for any of the aforementioned systems is likely to be the peristaltic pump.
4.2 Experimental setup considerations
When any of these serial systems are used for culturing experiments, the accumulation and influence of metabolites should be considered as seawater passes sequentially from one system chamber to the next, where the impact would be greatest in the last system chamber. Limiting such effects, which is important with very sensitive foraminiferal species, can be achieved by using fewer specimens in the series through the reduction of system chambers and/or quantity in the groups. Metabolites, however, may have a nominal impact on the results when single benthic foraminifera or a small group of benthic foraminifera more tolerant of highly variable conditions, such as A. tepida (e.g., Bradshaw, 1957; LeKieffre et al., 2017), are placed in individual chambers (depending on chamber volume relative to that of the foraminifera, flow rate, temperature, starting gas solubility, etc.). Furthermore, the potential impact on results from the feeding technique implemented (e.g., stress on the foraminifera or contamination) should also be considered. Dissolved oxygen measurements may be required for experiments conducted over longer time periods or using more system chambers per series.
Historically, and in the setup here, the systems are meant to be completed in series, as more than one chamber is used for a specific elemental concentration; thus, seawater composition steadiness might be more important at low concentrations. The seawater exposed to the foraminifera in the first chamber will vary in composition as it passes through other chambers with foraminifera. That is, the trace metal concentration may change, and the composition of other chemical species in the seawater (e.g., lower O2 and higher CO2 from aerobic respiration) will change as the seawater moves in series from one chamber to the next. Larger chambers, fewer individuals per chamber, and/or higher flow rate could reduce metabolite effects that likely need to be calculated for species-specific impacts. To evaluate the impact of metabolites (i.e., the change in chemical species available to the foraminifera), measurements from individual chambers should be compared. In addition, a sample of source seawater from the beginning of the experiment could be compared to the final output, along with output collected at periodic intervals (e.g., weekly).
Importantly, the configuration of System C does not have to be connected in series. Experiments can be performed in many different configurations in which each PFA vial could be isolated. Consequently, trace metal concentrations should remain steady and the impact of metabolites should be minimized in such an arrangement.
4.3 Selection of a serial flow-through culturing system
For the trial with 5 × [Mo]sw, the three culturing systems may have had some minor variability prior to the 6 h mark, but all stabilized no later than that point. System C was the best-performing system in the first trial with 2 × [Mo]sw, and additional measurements indicated that it stabilized within 2 h. System B was the next best with three measurements that diverged beyond the 268–299 nM range of the source seawater, while System A had measurements below the 95 % confidence interval of the source seawater (i.e., 268–306 nM) from the 60 h mark to the 108 h mark. For our Mo-uptake experiments, System C was selected because of its speed to reach equilibrium and consistency with both the lower and higher [Mo]sw tested.
Trace elements presumably have different saturation limits and affinities to adsorb onto surfaces; however, equilibrium may be reached more rapidly for higher concentrations because the adsorptive surfaces are exposed to higher elemental densities, whereas equilibration may take longer because more time is needed for adsorptive surfaces to reach saturation at lower concentrations. Although the systems had flat chamber bottoms and stratification of the source seawater was avoided by using two different lengths of tubes per chamber as illustrated in Fig. 2, round or conically shaped chamber bottoms could improve mixing in the chamber due to their concave form. A synopsis of assessments for each system is provided in Table 1.
Table 1Summary of advantages and disadvantages of System A using a plastic cell culture plate, System B utilizing a customized block of PTFE, and System C implementing a serial system of PFA vials. Constructive aspects are indicated by a check mark “✓”, neutral evaluations are marked with an open circle “∘”, and shortcomings are indicated by a cross “×”.
4.4 Molybdenum uptake into benthic foraminiferal calcite
Both experiments demonstrated that foraminifera incorporated Mo into their calcite tests with differences among the various [Mo]sw. Notably, differences between 5× and 10 × [Mo]sw in the second experiment may be statistically significant, but, when the seawater Mo concentrations were closer to one another (e.g., 1× versus 2 × [Mo]sw in the first experiment), overlap of standard deviation error bars suggests that the differences between those data sets are not or likely not statistically significant. In the initial and proof-of-concept foraminiferal uptake experiment with Mo concentrations of 1×, 2×, and 3 × [Mo]sw, the data were in a narrow range of approximately 0.0–0.6. The range of each treatment of [Mo]sw (mean ± 1 standard deviation and/or interquartile range) overlapped with the adjacent range(s). However, there was no overlap in the range of data between 1× and 3 × [Mo]sw. In the second experiment, this was assessed further for reproducibility and to test a wider range of concentrations for potential relationships. Importantly, the low measurements in the first foraminiferal uptake experiment demonstrated the importance of stabilizing the levels of redox-sensitive trace elements like Mo, as fluctuations could easily complicate the results and indicated that the lower the concentration, the more important adsorption onto the experimental setup could be.
In comparison to the previous experiment, the ratios for 1× and 3 × [Mo]sw in the second experiment were in a slightly wider range of approximately 0.2–0.5 and 0.3–1.2 , respectively. However, for the first and second experiments, the mean for 1 × [Mo]sw was 0.09 and 0.40 and the mean for 3 × [Mo]sw was 0.41 and 0.85 , respectively. In the second experiment, a measurement for Mo at 3 × [Mo]sw was exceptionally low compared to the [Ca] measurements. If this measurement was discarded, the mean for 3 × [Mo]sw in the second experiment would increase from 0.85 to 1.15 . Between the experiments, there is a difference of approximately 4 times among the 1 × [Mo]sw means. Similarly, there is a difference of about 2 times among the 3 × [Mo]sw means. Some of the low [Mo] values may be due to differences in the samples between the first and second experiments (e.g., average starting size of the juvenile foraminifera) and seem to lead to a larger range of ratios. As expected, there is less variability for samples with increased , which suggests that, as [Mo]sw increases, there is more consistent uptake. Further experiments that implement more precise controls on factors that have some variability should elucidate additional challenges and lead to greater consistency at these lower [Mo]sw values.
The concentrations tested in the second experiment were also further apart, and, because it had two higher concentrations (i.e., 5× and 10 × [Mo]sw), the complete range was about 0.2–7.7. Unlike the first experiment, in which there was a gap between 1× and 3 × [Mo]sw, the range of each [Mo]sw overlapped with the adjacent range(s) for all compositions except 10 × [Mo]sw. That is, there was an overlap between adjacent ranges of 1×, 3×, and 5 × [Mo]sw. However, one measurement for Mo for 3 × [Mo]sw and another for 5 × [Mo]sw were exceptionally low compared to corresponding measurements, so the ratio values were very low despite associated Ca measurements being within the target range of 15–25 ppm. Nonetheless, there was a considerable separation of ranges between 5× and 10 × [Mo]sw. Furthermore, if those two possible outliers were discarded, there would have been separation of all ranges between compositions of 1×, 3×, 5×, and 10 × [Mo]sw. Alternatively, if the data from 3 × [Mo]sw were discarded, there also would have been a greater separation between the other [Mo]sw. In both scenarios, the lack of overlap between adjacent error bars of seawater Mo concentrations would indicate a greater likelihood that differences in the data are statistically significant. Thus, System C, the experimental design using PFA vials and PTFE tubing, will provide new avenues of research due to its ability to help maintain trace element concentrations.
Three serial flow-through systems (A, B, and C) were reviewed that can be used for culturing experiments with benthic foraminifera, each having their benefits and drawbacks. These results from [Mo]sw demonstrate that, even though the three systems reached equilibrium early in the process when the source seawater was elevated to 5 × [Mo]sw, equilibration time varied for each device when the Mo seawater concentration was closer to that of natural seawater at 2 × [Mo]sw. In both cases, however, measurements from System C suggest it performed best, as it reached equilibrium with [Mo]sw within hours. Notably, System C did not deviate from the 95 % confidence interval of the source seawater for all periods in both experiments. Therefore, it is posited that lower concentrations of most elements are also reasonably expected to reach equilibrium faster with designs that use a significant amount of PTFE, PFA, and other adsorption-resistant materials.
Using the new experimental design with PTFE and PFA materials, Mo was tested at near-seawater concentrations, which were much lower than previously published experimental data on comparable redox-sensitive transition metals. These initial tests utilizing 1×, 2×, 3×, 5×, and 10 × [Mo]sw indicated that additional and more precise data are needed to elucidate the minor differences in the data at lower concentrations. The second experiment also showed that utilizing larger spreads between trace element concentrations could provide more discernable results. Lastly, these foraminiferal uptake experiments indicate that there are statistical differences between near-modern concentrations for Mo and that there will likely be similar differences for other trace elements, which could provide new insights for a range of modern and ancient seawater concentrations.
It is also postulated from the 5 × [Mo]sw results, and from foraminiferal calcite data of the second experiment at 5× and 10 × [Mo]sw, that very high amounts of a trace element are unlikely to pose an issue due to adsorption by any of the aforementioned devices; however, the amount of time for a serial flow-through system to reach equilibrium with its source seawater can only be attained by trial. Future modifications could improve their utility or reliability. As more foraminiferal calcite data for trace elements in ppb or lower quantities from ICP-MS become attainable, the element itself and the amounts to be tested must be assessed when determining the optimal approach that does not impact uptake by the organisms studied.
For future research, System C is likely the best selection for trace metal uptake experiments due to consistency (as shown by its low standard deviation in both [Mo]sw experiments), interchangeable parts, and configuration flexibility. These results advocate incorporating fluoropolymer products when conducting trials that culture calcifying organisms to study their incorporation of trace metals. We expect dependable results from our subsequent experiments that will utilize living benthic foraminifera to measure the uptake of redox-sensitive trace metals. More broadly, experimental designs for low oceanic elemental concentrations should benefit from the utilization of more adsorption-resistant materials.
Table A1Data and statistics of the source seawater and three systems tested for the time to reach equilibrium using 5 × [Mo]sw.
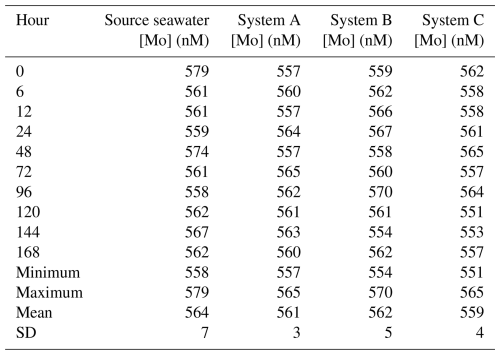
Table A2Data and statistics of the source seawater and three systems tested for the time to reach equilibrium using 2 × [Mo]sw.
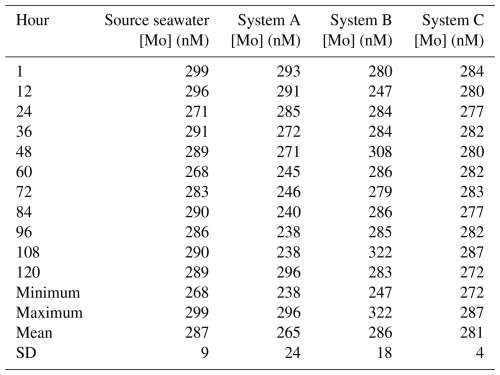
Table A3Data and statistics of foraminiferal calcite where A. tepida was exposed to seawater at 1×, 2×, and 3 × [Mo]sw. Quartiles were calculated by the inclusive method when there were only two values.
All data sets discussed in the article are available in Appendix A.
Samples were collected by CHG and analyzed by CHG and JDO. The initial draft was written by CHG, with subsequent revisions and interpretations by all authors. The submitted draft was collaboratively written by CHG, JDO, and MMC.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
This article is part of the special issue “Advances and challenges in modern and benthic foraminifera research: a special issue dedicated to Professor John Murray”. It is not associated with a conference.
For their contributions, we thank Datu Adiatma, John Goodin, Lisa Keith (née Guy), Sean Newby, Michael Rimmel, and Seth A. Young. Financial support was provided by the Cushman Foundation for Foraminiferal Research through the Loeblich and Tappan Student Research Award. Additional financial support was provided by Mr. and Mrs. Helmut Gfatter, Florida State University, and Phi Kappa Phi. A portion of this work was performed at the National High Magnetic Field Laboratory, which is supported by the National Science Foundation Cooperative Agreement nos. DMR-1644779 and DMR-2128556 and the State of Florida. Furthermore, Jeremy D. Owens thanks the National Science Foundation, National Aeronautics and Space Administration, and the Sloan Foundation for funding ICP-MS work on the elements. We also thank an anonymous reviewer, who provided multiple critiques, and the editors for their contributions to the article. This article is dedicated to the memory of John Murray and is emblematic of his book, “Ecology and Applications of Benthic Foraminifera”, which has been and continues to be as important resource for numerous foraminiferal researchers.
The ICP-MS work on the elements conducted at the National High Magnetic Field Laboratory – supported by the National Science Foundation Cooperative Agreement nos. DMR-1644779 and DMR-2128556 and the State of Florida – was partially funded by the National Science Foundation (grant no. EAR2026926), the National Aeronautics and Space Administration (grant nos. NNX16AJ60G and 80NSSC18K1532), and the Sloan Foundation (grant no. FG-2020-13552).
This paper was edited by Irina Polovodova Asteman and reviewed by Lennart de Nooijer and one anonymous referee.
Algeo, T. J. and Lyons, T. W.: Mo–total organic carbon covariation in modern anoxic marine environments: Implications for analysis of paleoredox and paleohydrographic conditions, Paleoceanography, 21, https://doi.org/10.1029/2004PA001112, 2006.
Algeo, T. J. and Maynard, J. B.: Trace-element behavior and redox facies in core shales of Upper Pennsylvanian Kansas-type cyclothems, Chemical Geology, 206, 289–318, https://doi.org/10.1016/j.chemgeo.2003.12.009, 2004.
Allen, K. A., Hönisch, B., Eggins, S. M., Haynes, L. L., Rosenthal, Y., and Yu, J.: Trace element proxies for surface ocean conditions: A synthesis of culture calibrations with planktic foraminifera, Geochimica et Cosmochimica Acta, 25, https://doi.org/10.1016/j.gca.2016.08.015, 2016.
Barras, C., Mouret, A., Nardelli, M. P., Metzger, E., Petersen, J., La, C., Filipsson, H. L., and Jorissen, F.: Experimental calibration of manganese incorporation in foraminiferal calcite, Geochim. Cosmochim. Act., 237, 49–64, https://doi.org/10.1016/j.gca.2018.06.009, 2018.
Bernhard, J. M., Blanks, J. K., Hintz, C. J., and Chandler, G. T.: Use of the fluorescent calcite marker calcein to label foraminiferal tests, J. Foramin. Res., 34, 96–101, https://doi.org/10.2113/0340096, 2004.
Bowman, C. N., Lindskog, A., Kozik, N. P., Richbourg, C. G., Owens, J. D., and Young, S. A.: Integrated sedimentary, biotic, and paleoredox dynamics from multiple localities in southern Laurentia during the late Silurian (Ludfordian) extinction event, Palaeogeogr. Palaeocl., 553, 109799, https://doi.org/10.1016/j.palaeo.2020.109799, 2020.
Bradshaw, J. S.: Laboratory studies on the rate of growth of the foraminifer, “Streblus beccarii (Linné) var. tepida (Cushman)”, J. Paleontol., 31, 1138–1147, https://www.jstor.org/stable/1300499 (last access: 1 October 2024), 1957.
Bruland, K. W., Donat, J. R., and Hutchins, D. A.: Interactive influences of bioactive trace metals on biological production in oceanic waters, Limnol. Oceanogr., 36, 1555–1577, https://doi.org/10.4319/lo.1991.36.8.1555, 1991.
Bruland, K. W., Middag, R., and Lohan, M. C.: 8.2 – Controls of Trace Metals in Seawater, in: Treatise on Geochemistry, 2nd Edn., edited by: Holland, H. D. and Turekian, K. K., Elsevier, Oxford, 19–51, https://doi.org/10.1016/B978-0-08-095975-7.00602-1, 2014.
Chandler, G. T., Williams, D. F., Spero, H. J., and Xiaodong, G.: Sediment microhabitat effects on carbon stable isotopic signatures of microcosm-cultured benthic foraminifera, Limnol. Oceanogr., 41, 680–688, https://doi.org/10.4319/lo.1996.41.4.0680, 1996.
Chappell, J. and Shackleton, N. J.: Oxygen isotopes and sea level, Nature, 324, 137–140, https://doi.org/10.1038/324137a0, 1986.
Collier, R. W.: Molybdenum in the Northeast Pacific Ocean, Limnol. Oceanogr., 30, 1351–1354, https://doi.org/10.4319/lo.1985.30.6.1351, 1985.
de Nooijer, L. J., Reichart, G. J., Dueñas-Bohórquez, A., Wolthers, M., Ernst, S. R., Mason, P. R. D., and van der Zwaan, G. J.: Copper incorporation in foraminiferal calcite: results from culturing experiments, Biogeosciences, 4, 493–504, https://doi.org/10.5194/bg-4-493-2007, 2007.
Delaney, M. L., Bé, A. W. H., and Boyle, E. A.: Li, Sr, Mg, and Na in foraminiferal calcite shells from laboratory culture, sediment traps, and sediment cores, Geochim. Cosmochim. Act., 49, 1327–1341, https://doi.org/10.1016/0016-7037(85)90284-4, 1985.
Dickson, A. J., Jenkyns, H. C., Porcelli, D., van den Boorn, S., and Idiz, E.: Basin-scale controls on the molybdenum-isotope composition of seawater during Oceanic Anoxic Event 2 (Late Cretaceous), Geochim. Cosmochim. Act., 178, 291–306, https://doi.org/10.1016/j.gca.2015.12.036, 2016.
Dissard, D., Nehrke, G., Reichart, G. J., and Bijma, J.: The impact of salinity on the and ratio in the benthic foraminifera Ammonia tepida: Results from culture experiments, Geochimica et Cosmochimica Acta, 74, 928–940, https://doi.org/10.1016/j.gca.2009.10.040, 2010.
Diz, P., Mena, A., Nombela, M. A., Castano-Carrera, M., Velo, A., Ordonez, N., Fuentes-Lema, A., Frances, G., Roson, G., Perez, F. F., and Rios, A. F.: Description of an experimental set up for the culture of benthic foraminifera in controlled pH conditions, Thalassas, 31, 23–32, 2015.
Emiliani, C.: Pleistocene temperatures, J. Geol., 63, 538–578, https://doi.org/10.1086/626295, 1955.
Filipsson, H. L., Bernhard, J. M., Lincoln, S. A., and McCorkle, D. C.: A culture-based calibration of benthic foraminiferal paleotemperature proxies: δ18O and results, Biogeosciences, 7, 1335–1347, https://doi.org/10.5194/bg-7-1335-2010, 2010.
Gfatter, C. and Owens, J. D.: Assessing trace metal incorporation using a flow through culturing system for benthic foraminifera, 2021 GSA Annual Meeting, Portland, 13 October 2021, Geological Society of America, https://doi.org/10.1130/abs/2021AM-370900, 2021.
Gill, B. C., Lyons, T. W., Young, S. A., Kump, L. R., Knoll, A. H., and Saltzman, M. R.: Geochemical evidence for widespread euxinia in the Later Cambrian ocean, Nature, 469, 80–83, https://doi.org/10.1038/nature09700, 2011.
Havach, S. M., Chandler, G. T., Wilson-Finelli, A., and Shaw, T. J.: Experimental determination of trace element partition coefficients in cultured benthic foraminifera, Geochim. Cosmochim. Act., 65, 1277–1283, https://doi.org/10.1016/S0016-7037(00)00563-9, 2001.
Hemleben, C., Spindler, M., and Anderson, O. R.: Modern Planktonic Foraminifera, Springer-Verlag, New York, https://doi.org/10.1007/978-1-4612-3544-6, 1989.
Hester, K. and Boyle, E.: Water chemistry control of cadmium content in Recent benthic foraminifera, Nature, 298, 260–262, https://doi.org/10.1038/298260a0, 1982.
Hintz, C. J., Chandler, G. T., Bernhard, J. M., McCorkle, D. C., Havach, S. M., Blanks, J. K., and Shaw, T. J.: A physicochemically constrained seawater culturing system for production of benthic foraminifera, Limnol. and Oceanogr.-Meth., 2, 160–170, https://doi.org/10.4319/lom.2004.2.160, 2004.
Hintz, C. J., Shaw, T. J., Bernhard, J. M., Chandler, G. T., McCorkle, D. C., and Blanks, J. K.: Trace/minor element : calcium ratios in cultured benthic foraminifera. Part II: Ontogenetic variation, Geochim. Cosmochim. Act., 70, 1964–1976, https://doi.org/10.1016/j.gca.2005.12.019, 2006a.
Hintz, C. J., Shaw, T. J., Chandler, G. T., Bernhard, J. M., McCorkle, D. C., and Blanks, J. K.: Trace/minor element: calcium ratios in cultured benthic foraminifera. Part I: Inter-species and inter-individual variability, Geochimica Et Cosmochimica Acta, 70, 1952–1963, https://doi.org/10.1016/j.gca.2005.12.018, 2006b.
Katz, M. E., Cramer, B. S., Franzese, A., Honisch, B., Miller, K. G., Rosenthal, Y., and Wright, J. D.: Traditional and Emerging Geochemical Proxies in Foraminifera, J. Foramin. Res., 40, 165–192, https://doi.org/10.2113/gsjfr.40.2.165, 2010.
Kendall, B., Dahl, T. W., and Anbar, A. D.: The stable isotope geochemistry of molybdenum, Rev. Mineral. Geochem., 82, 683–732, https://doi.org/10.2138/rmg.2017.82.16, 2017.
Kozik, N. P., Gill, B. C., Owens, J. D., Lyons, T. W., and Young, S. A.: Geochemical Records Reveal Protracted and Differential Marine Redox Change Associated With Late Ordovician Climate and Mass Extinctions, AGU Advances, 3, e2021AV000563, https://doi.org/10.1029/2021AV000563, 2022.
Kramar, U., Munsel, D., Berner, Z., Bijma, J., and Nehrke, G.: Determination Of Trace Element Incorporation Into Tests Of In Vitro Grown Foraminifera By Micro- SYXRF – A Basis For The Development Of Paleoproxies, AIP Conf. Proc., 1221, 154–159, https://doi.org/10.1063/1.3399243, 2010.
Lea, D. W.: Trace elements in foraminiferal calcite, in: Modern Foraminifera, edited by: Sen Gupta, B. K., Kluwer Academic Publishers, Dordrecht, 259–277, https://doi.org/10.1007/0-306-48104-9_15, 1999.
Lea, D. W. and Boyle, E. A.: Barium content of benthic foraminifera controlled by bottom-water composition, Nature, 338, 751–753, https://doi.org/10.1038/338751a0, 1989.
Lea, D. W. and Spero, H. J.: Experimental determination of barium uptake in shells of the planktonic foraminifera Orbulina universa at 22 °C, Geochim. Cosmochim. Act., 56, 2673–2680, https://doi.org/10.1016/0016-7037(92)90352-J, 1992.
Lea, D. W. and Spero, H. J.: Assessing the reliability of paleochemical tracers: Barium uptake in the shells of planktonic foraminifera, Paleoceanography, 9, 445–452, https://doi.org/10.1029/94pa00151, 1994.
Lea, D. W., Mashiotta, T. A., and Spero, H. J.: Controls on magnesium and strontium uptake in planktonic foraminifera determined by live culturing, Geochim. Cosmochim. Act., 63, 2369–2379, https://doi.org/10.1016/S0016-7037(99)00197-0, 1999.
Lea, D. W., Pak, D. K., and Spero, H. J.: Climate Impact of Late Quaternary Equatorial Pacific Sea Surface Temperature Variations, Science, 289, 1719–1724, https://doi.org/10.1126/science.289.5485.1719, 2000.
Lear, C. H., Elderfield, H., and Wilson, P. A.: Cenozoic Deep-Sea Temperatures and Global Ice Volumes from in Benthic Foraminiferal Calcite, Science, 287, 269–272, https://doi.org/10.1126/science.287.5451.269, 2000.
Lear, C. H., Rosenthal, Y., and Slowey, N.: Benthic foraminiferal Mg/Ca-paleothermometry: a revised core-top calibration, Geochim. Cosmochim. Act., 66, 3375–3387, https://doi.org/10.1016/S0016-7037(02)00941-9, 2002.
Le Cadre, V. and Debenay, J.-P.: Morphological and cytological responses of Ammonia (foraminifera) to copper contamination: Implication for the use of foraminifera as bioindicators of pollution, Environ. Pollut., 143, 304–317, https://doi.org/10.1016/j.envpol.2005.11.033, 2006.
LeKieffre, C., Spangenberg, J. E., Mabilleau, G., Escrig, S., Meibom, A., and Geslin, E.: Surviving anoxia in marine sediments: The metabolic response of ubiquitous benthic foraminifera (Ammonia tepida), PLOS ONE, 12, 1–21, https://doi.org/10.1371/journal.pone.0177604, 2017.
Lowery, C. M., Bralower, T. J., Owens, J. D., Rodríguez-Tovar, F. J., Jones, H., Smit, J., Whalen, M. T., Claeys, P., Farley, K., Gulick, S. P. S., Morgan, J. V., Green, S., Chenot, E., Christeson, G. L., Cockell, C. S., Coolen, M. J. L., Ferrière, L., Gebhardt, C., Goto, K., Kring, D. A., Lofi, J., Ocampo-Torres, R., Perez-Cruz, L., Pickersgill, A. E., Poelchau, M. H., Rae, A. S. P., Rasmussen, C., Rebolledo-Vieyra, M., Riller, U., Sato, H., Tikoo, S. M., Tomioka, N., Urrutia-Fucugauchi, J., Vellekoop, J., Wittmann, A., Xiao, L., Yamaguchi, K. E., and Zylberman, W.: Rapid recovery of life at ground zero of the end-Cretaceous mass extinction, Nature, 558, 288–291, https://doi.org/10.1038/s41586-018-0163-6, 2018.
Lu, Z., Jenkyns, H. C., and Rickaby, R. E. M.: Iodine to calcium ratios in marine carbonate as a paleo-redox proxy during oceanic anoxic events, Geology, 38, 1107–1110, https://doi.org/10.1130/g31145.1, 2010.
Martínez-Colón, M.: Pollutants and Foraminiferal Assemblages in Torrecillas Lagoon: An Environmental Micropaleontology Approach, Doctoral dissertation, College of Marine Science, University of South Florida, 238 pp., https://digitalcommons.usf.edu/etd/6307 (last access: 1 July 2025), 2016.
Martínez-Colón, M., Hallock, P., Green-Ruíz, C. R., and Smoak, J. M.: Benthic foraminifera as bioindicators of potentially toxic element (PTE) pollution: Torrecillas lagoon (San Juan Bay Estuary), Puerto Rico, Ecol. Indic., 89, 516–527, https://doi.org/10.1016/j.ecolind.2017.10.045, 2018.
Mashiotta, T. A., Lea, D. W., and Spero, H. J.: Experimental determination of cadmium uptake in shells of the planktonic foraminifera Orbulina universa and Globigerina bulloides: Implications for surface water paleoreconstructions, Geochim. Cosmochim. Act., 61, 4053-4065, https://doi.org/10.1016/s0016-7037(97)00206-8, 1997.
McCorkle, D. C., Martin, P. A., Lea, D. W., and Klinkhammer, G. P.: Evidence of a dissolution effect on benthic foraminiferal shell chemistry: ä13C, , , and results from the Ontong Java Plateau, Paleoceanography, 10, 699–714, https://doi.org/10.1029/95pa01427, 1995.
Meilland, J., Siccha, M., Morard, R., and Kucera, M.: Continuous reproduction of planktonic foraminifera in laboratory culture, J. Eukaryot. Microbiol., 71, e13022, https://doi.org/10.1111/jeu.13022, 2024.
Miller, C. A., Peucker-Ehrenbrink, B., Walker, B. D., and Marcantonio, F.: Re-assessing the surface cycling of molybdenum and rhenium, Geochim. Cosmochim. Act., 75, 7146–7179, https://doi.org/10.1016/j.gca.2011.09.005, 2011.
Miller, K. G., Wright, J. D., Katz, M. E., Wade, B. S., Browning, J. V., Cramer, B. S., and Rosenthal, Y.: Climate threshold at the Eocene–Oligocene transition: Antarctic ice sheet influence on ocean circulation, in: The Late Eocene Earth – Hothouse, Icehouse, and Impacts, edited by: Koeberl, C. and Montanari, A., Geological Society of America Special Papers, Geological Society of America, 169–178, https://doi.org/10.1130/2009.2452(11), 2009.
Morford, J. L. and Emerson, S.: The geochemistry of redox sensitive trace metals in sediments, Geochim. Cosmochim. Act., 63, 1735–1750, https://doi.org/10.1016/s0016-7037(99)00126-x, 1999.
Morse, J. W. and Bender, M. L.: Partition coefficients in calcite: Examination of factors influencing the validity of experimental results and their application to natural systems, Chem. Geol., 82, 265–277, https://doi.org/10.1016/0009-2541(90)90085-L, 1990.
Munsel, D., Kramar, U., Dissard, D., Nehrke, G., Berner, Z., Bijma, J., Reichart, G.-J., and Neumann, T.: Heavy metal incorporation in foraminiferal calcite: results from multi-element enrichment culture experiments with Ammonia tepida, Biogeosciences, 7, 2339–2350, https://doi.org/10.5194/bg-7-2339-2010, 2010.
Murray, J. W.: Ecology and Applications of Benthic Foraminifera, Cambridge University Press, Cambridge, 426 pp., https://doi.org/10.1017/CBO9780511535529, 2006.
Owens, J. D., Reinhard, C. T., Rohrssen, M., Love, G. D., and Lyons, T. W.: Empirical links between trace metal cycling and marine microbial ecology during a large perturbation to Earth's carbon cycle, Earth Planet. Sc. Lett., 449, 407–417, https://doi.org/10.1016/j.epsl.2016.05.046, 2016.
Polovodova Asteman, I., Hanslik, D., and Nordberg, K.: An almost completed pollution-recovery cycle reflected by sediment geochemistry and benthic foraminiferal assemblages in a Swedish–Norwegian Skagerrak fjord, Mar. Pollut. Bull., 95, 126–140, https://doi.org/10.1016/j.marpolbul.2015.04.031, 2015.
Reinhard, C. T., Planavsky, N. J., Robbins, L. J., Partin, C. A., Gill, B. C., Lalonde, S. V., Bekker, A., Konhauser, K. O., and Lyons, T. W.: Proterozoic ocean redox and biogeochemical stasis, P. Natl. Acad. Sci. USA, 110, 5357–5362, https://doi.org/10.1073/pnas.1208622110, 2013.
Richirt, J., Schweizer, M., Bouchet, V. M. P., Mouret, A., Quinchard, S., and Jorissen, F. J.: Morphological Distinction of Three Ammonia Phylotypes Occurring Along European Coasts, J. Foramin. Res., 49, 76–93, https://doi.org/10.2113/gsjfr.49.1.76, 2019.
Rosenthal, Y., Boyle, E. A., and Slowey, N.: Temperature control on the incorporation of magnesium, strontium, fluorine, and cadmium into benthic foraminiferal shells from Little Bahama Bank: Prospects for thermocline paleoceanography, Geochim. Cosmochim. Act., 61, 3633–3643, https://doi.org/10.1016/S0016-7037(97)00181-6, 1997.
Schiebel, R. and Hemleben, C.: Modern planktic foraminifera, Palaont. Z., 79, 135–148, https://doi.org/10.1007/BF03021758, 2005.
Schmidt, S., Hathorne, E. C., Schönfeld, J., and Garbe-Schönberg, D.: Heavy metal uptake of nearshore benthic foraminifera during multi-metal culturing experiments, Biogeosciences, 19, 629–664, https://doi.org/10.5194/bg-19-629-2022, 2022.
Shackleton, N. J.: Oxygen isotope stratigraphic record of Late Pleistocene, Philos. T. Roy. Soc. B., 280, 169–182, https://doi.org/10.1098/rstb.1977.0104, 1977.
Smith, C. W., Fehrenbacher, J. S., and Goldstein, S. T.: Incorporation of heavy metals in experimentally grown foraminifera from Sapelo Island, Georgia and Little Duck Key, Florida, U.S.A, Mar. Micropaleontol., 156, 101854, https://doi.org/10.1016/j.marmicro.2020.101854, 2020.
Them II, T. R., Owens, J. D., Marroquín, S. M., Caruthers, A. H., Alexandre, J. P. T., and Gill, B. C.: Reduced Marine Molybdenum Inventory Related to Enhanced Organic Carbon Burial and an Expansion of Reducing Environments in the Toarcian (Early Jurassic) Oceans, AGU Advances, 3, e2022AV000671, https://doi.org/10.1029/2022AV000671, 2022.
van Dijk, I., Mouret, A., Cotte, M., Le Houedec, S., Oron, S., Reichart, G.-J., Reyes-Herrera, J., Filipsson, H., L, and Barras, C.: Chemical Heterogeneity of Mg, Mn, Na, S, and Sr in Benthic Foraminiferal Calcite, Front. Earth Sci., 1, https://doi.org/10.3389/feart.2019.00281, 2019.
Westgård, A., Ezat, M. M., Chalk, T. B., Chierici, M., Foster, G. L., and Meilland, J.: Large-scale culturing of Neogloboquadrina pachyderma, its growth in, and tolerance of, variable environmental conditions, J. Plankton Res., 45, 732–745, https://doi.org/10.1093/plankt/fbad034, 2023.
Wilson-Finelli, A., Chandler, G. T., and Spero, H. J.: Stable isotope behavior in paleoceanographically important benthic Foraminifera; results from microcosm culture experiments, J. Foramin. Res., 28, 312–320, 1998.
Zachos, J. C., Stott, L. D., and Lohmann, K. C.: Evolution of Early Cenozoic marine temperatures, Paleoceanography, 9, 353–387, https://doi.org/10.1029/93PA03266, 1994.