the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Quantitative measurement of benthic foraminifera sediment reworking using a three-dimensional sensor
Manon Doutrelant
Alicia Romero-Ramirez
Aurélie Ciutat
Vincent M. P. Bouchet
Olivier Maire
Despite their worldwide distribution and very high densities, the contribution of benthic meiofaunal species to sediment reworking has largely been neglected in bioturbation research. This is partly due to the challenge in obtaining reliable measurements of these minute size organisms. So far, only a handful of studies have investigated the influence of these microbioturbators on particle transport processes at the sediment surface. These studies most often used the surface image analysis (SIA) method, which indirectly estimates surface sediment reworking rate (SSRR) by tracking the position of individuals at the sediment surface over time. Here, focusing on benthic foraminifera, we demonstrate that successive assessments of sediment microtopography mapping using a three-dimensional (3D) sensor can provide direct and accurate quantifications of meiofaunal SSRR, with high spatial and temporal resolutions. This new method is thus particularly suitable to investigating the as-yet-unknown influence of the meiobenthic fauna, such as foraminifera, on particle transport at the sediment–water interface and more generally on the functioning of benthic soft-bottom ecosystems.
- Article
(5867 KB) - Full-text XML
-
Supplement
(360 KB) - BibTeX
- EndNote
The bioturbation of aquatic soft-bottom substrates is commonly defined as the mixing of both particles (i.e. sediment reworking) and porewater solutes (i.e. bioirrigation) by benthic organisms (Shull et al., 2009; Kristensen et al., 2012). It occurs in almost all marine sedimentary environments, although it is usually more intense in productive shallow coastal areas inhabited by diversified and abundant infaunal communities than in the deep ocean (Buffoni et al., 1992; Henderson et al., 1999; Kim and Burnett, 1988). In cohesive sediments, bioturbation is the dominant mode of particulate and solute transport, which profoundly influences their physical (e.g. grain size, porosity, permeability, surface roughness), chemical (e.g. O2 penetration depth and the spatiotemporal distribution of other electron acceptors), and biological (e.g. structure, diversity, and abundance of microbial communities) properties (Aller, 2014; Dairain et al., 2020; De Borger et al., 2020; Gautreau et al., 2023; Meadows and Tufail, 1986; Orvain et al., 2004). As a consequence, bioturbation controls numerous ecosystem processes and functions (e.g. microbial-mediated mineralization processes and nutrient fluxes across the sediment–water interface, which in turn sustains primary and secondary production) (Mermillod-Blondin et al., 2004; Richard et al., 2023; Shen et al., 2017).
The mode and the intensity of sediment bioturbation, and thus its overall influence on ecosystem functioning, can be seen as the interplay between body size, abundance, spatial distribution, and biological and functional traits of dominant species (Bernard et al., 2019; Kristensen et al., 2012; Solan et al., 2008). Accordingly, research on bioturbation has mainly focused on macrobenthic invertebrates (Queirós et al., 2013; Schratzberger and Ingels, 2018), which generally exhibit high densities over large spatial scales and are thus considered the most effective contributors despite an individual bioturbation potential (i.e. the capacity of a single organism to contribute to sediment bioturbation processes) which is moderate (e.g. 163 mm3 ind−1 d−1 for the polychaeta Melinna palmata; Massé et al., 2019) compared to megafaunal species, for instance (e.g. 81 m3 ind−1 d−1 for the Grey whale, Eschrichtius robustus; Nelson et al., 1987). In contrast, due to their minute size (63–1000 µm) and a low individual bioturbation potential (e.g. 21.6 mm3 ind−1 d−1 for the foraminifera Quinqueloculina seminulum; Deldicq et al., 2021), the influence of meiofauna on bioturbation processes has long been considered of limited importance and thus poorly studied (see review in Schratzberger and Ingels, 2018). However, meiofaunal communities are widespread (i.e. from intertidal to deep-sea ecosystems in all polar, temperate, and tropical environments), abundant (i.e. averaging millions of individuals per square metre; Coull, 1999), and diverse (Murray, 2007). They can even be dominant in some extreme environments (see review in Zeppilli et al., 2018). Moreover, several species, such as nematodes and foraminifera, are known to continuously rework the sediment surface and thus have the potential to significantly influence benthic ecosystem processes and functions, including microbial respiration, nutrient cycling, and the flow of energy at the sediment–water interface (Bonaglia et al., 2014, 2020; Langlet et al., 2023).
In intertidal environments, recent studies even suggested that the contribution of meiofaunal communities to particle mixing could be of the same order of magnitude as those of macrofauna (Bouchet and Seuront, 2020; Gross, 2002). However, most of these studies are merely based on visual observations (Severin et al., 1982) or gross estimations (Bouchet and Seuront, 2020; Deldicq et al., 2021; Gross, 2002) of the amount of sediment displaced through locomotion and did not rigorously measure the total sediment reworking rate resulting from all biological activities (e.g. locomotion, feeding, burrowing, cyst or tube construction). One reason for this is that the current methods used to quantify total sediment reworking rates (i.e. involving particle-tracers) have been specifically designed to assess vertical transport generated by large organisms over relatively thick sediment layers (>5 mm) (Maire et al., 2008). Therefore, they cannot easily be implemented in meiofaunal studies where the mixed layer depth is much lower (i.e. <5 mm). As a consequence, mostly surface image analysis (SIA) has been used so far to estimate the bioturbation activity of meiofaunal species such as foraminifera (Bouchet and Seuront, 2020; Deldicq et al., 2021). More precisely, SIA consists of tracking the position of individuals through successive images to determine the characteristics of motion behaviour. The surface sediment reworking rate is then a posteriori estimated from the distance travelled by the organisms during a given period of time and their mean test surface (Bouchet and Seuront, 2020; Deldicq et al., 2021; Maire et al., 2008). As such, SIA is not a direct measurement of sediment reworking rate but an indirect estimation. In contrast, quantifying surface sediment reworking through successive microtopography mapping (Maire et al., 2007; Røy et al., 2002, 2005) seems particularly suitable to quantify the bioturbation activity of the meiobenthos living at the sediment–water interface. However, the two classical methods (i.e. the use of a laser line projected onto the sediment surface or a laser telemeter mounted on two crossed-step motor tables) have several technical limitations (e.g. no possible measurement behind mounds or within sharp pits, time needed to assess the microtopography) that drastically limit their spatial and temporal resolutions and thus their accuracy (see review in Maire et al., 2008). A new method, based on the use of a three-dimensional (3D) sensor, offers the potential to overcome these technical limitations and thus represents a promising alternative to derive surface sediment reworking rates from successive microtopography mapping with a high spatial resolution (i.e. vertical resolution < 35 µm). However, this method has yet to be implemented to quantify the bioturbation activity of meiobenthic organisms living at the sediment–water interface, such as benthic foraminifera.
In this context, the specific aims of this study were (1) to test the ability of the 3D sensor method to accurately quantify the sediment transport generated by meiobenthic organisms, such as benthic foraminifera, and (2) to compare our results to those obtained by SIA. The 3D microtopographic method was tested on the benthic foraminifera Ammonia confertitesta, a common and highly abundant benthic foraminiferal species inhabiting European temperate mudflats (Fouet et al., 2024a; Pavard et al., 2023). Species from the genus Ammonia have the ability through their pseudopodial activity and motion behaviour to induce particle transport at the sediment surface (Bouchet and Seuront, 2020; Chandler, 1989; Deldicq et al., 2021). The mode of locomotion of Ammonia spp., and of other benthic foraminifera, is based on an amoeboid movement (i.e. crawling-like type of displacement achieved by protrusion of cytoplasm and the formation of pseudopods). Pseudopods, which typically extend from a few millimetres up to several centimetres (Bernstein et al., 1978), are also involved in the construction of cysts (a secondary-like test build with sediment particles around the calcareous test; Cedhagen et al., 2021). During locomotion events, individuals leave their cyst (Heinz et al., 2005) and may later build a new one, thereby also leading to the transfer of sediment horizontal particles (Bouchet and Seuront, 2020; Deldicq et al., 2021; Cedhagen et al., 2021). Previous studies estimated that A. confertitesta can move at the sediment surface at a speed comprising between 17 and 70 mm d−1 (Bouchet and Seuront, 2020; Deldicq et al., 2021), therefore making this species a good model to compare the accuracy of both methods (i.e. SIA vs. 3D sensor) in quantifying benthic foraminiferal surface sediment reworking rates.
2.1 Sediment and foraminifera sampling
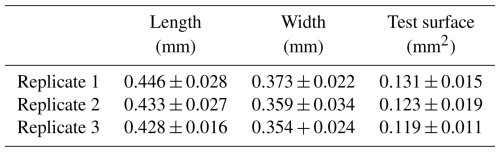
Surface sediment (0–1 cm) was collected at low tide in February 2024 at the Phare de Richard (45°26′383′′ N, 0°55′850′′ W), an intertidal mudflat located on the French Atlantic coast in the Gironde estuary. Samples were then sieved at the laboratory through a 125 µm mesh, and all specimens of Ammonia confertitesta were identified and sorted with a brush. To ensure their vitality, foraminifera were left for 30 min on a Petri dish with a thin layer of sieved sediment (size fraction < 63 µm). Only individuals that made a track at the sediment surface were considered alive. Living specimens were then photographed and sized (both length and width) with the Fiji software (https://imagej.net/software/fiji/, last access: 10 February 2025). Individuals with a length comprising between 400 and 500 µm (Table 1) were kept in seawater for 12 h in a controlled-temperature room at 19 °C before being used in the experiment.
2.2 Experimental setup and assessment of the sediment microtopography
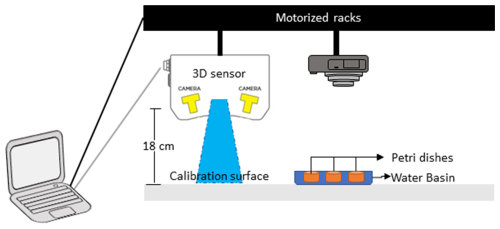
The experiment was conducted in a controlled-temperature room at 19 °C under constant white light exposure. Six Petri dishes (internal diameter of 8.7 cm) were filled with an 8 mm thick layer of natural muddy sediment, to mimic the optimal microhabitat of Ammonia confertitesta (i.e. ranging from 0 to 5 mm; Cesbron et al., 2016). The sediment was covered by a thin layer of natural filtered seawater (∼5 mm) to avoid desiccation during the 34 h of the experiment. The sediment was previously frozen at −20 °C during 48 h to eliminate all the living fauna and was defrosted 24 h before being used. At the beginning of the experiment, 15 individuals of A. confertitesta (corresponding to the natural density of this species; Fouet et al., 2024b) were deposited at the surface of each Petri dish (n=3) with a pipette. Three other Petri dishes were kept without foraminifera (= Control). Temporal changes in the sediment microtopography were then assessed using a 3D sensor (LMI Gocator 3110 sensor) placed on two crossed-step motorized racks (Fig. 1). This 3D sensor generates structured blue light (465 nm) to project onto the sediment surface. The reflection of this blue light is acquired by stereo cameras in the 3D sensor in order to create a 3D data in the shape of a 3D point cloud. The 3D sensor is placed 18 cm above the Petri dish, thus allowing the monitoring of the entire sediment surface with a high resolution (i.e. 100 µm for the XY dimensions and 35 µm for the Z dimension) (Fig. 1). The calibration was achieved on a flat surface prior to the first measurement. A clear picture of the sediment surface (SIA method) was also taken with a standard reflex camera (Canon EOS 500D). Acquisitions were made manually every hour following the introduction of foraminifera from 0 to 10 h and from 24 to 34 h (no acquisition during the night).
2.3 Calculation of sediment reworking rate using the 3D sensor
Microtopographic data obtained with the 3D sensor were analysed using a specific script developed with MATLAB (Fig. 2). The script starts by reading the 3D data (Fig. 2a) within a region of interest (ROI), which is manually selected (Fig. 2b). In this study, the ROI was defined as a rectangle of 55 mm by 55 mm that maximizes the surface of sediment used in the calculation of reworking rates, whilst excluding the border of the Petri dish. All individual displacements and surface sediment reworking recorded during the 34 h of the experiment were strictly limited to this area. To assess hourly sediment transport, the microtopography acquired at time Tn was subtracted to the microtopography acquired at time Tn+1 (Fig. 2c). Different types of noise can be present in our data mainly due to water movement and sediment compaction. This noise is invisible to the human eye but visible on the initial acquisition. In order to reduce these effects, the data are filtered on the Z dimension to eliminate any change in the range ±150 µm (Fig. 2d).
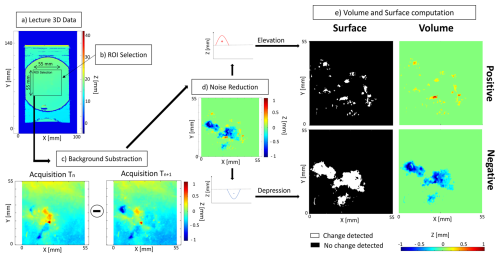
Figure 2Flowchart of the data acquisition using MATLAB to compute the volume and surface of sediment reworked. These different steps are (a) lecture of the 3D data (i.e. transformation of coordinate into a map), (b) extraction of the region of interest (ROI), (c) background subtraction (), (d) elimination of the noise on the Z axis, and (e) calculation of the four indices of sediment reworking.
Finally, four different indices of sediment reworking can be calculated (Fig. 2e): (1) the surface of sediment impacted by an elevation of the initial sediment–water interface (= Positive Surface), (2) the surface of sediment impacted by a depression of the initial sediment–water interface (= Negative Surface), (3) the total volume of sediment transported onto the initial sediment–water interface (= Positive Volume), and (4) the total volume of excavated sediment (= Negative Volume).
At each time, rates of surficial sediment reworking were calculated in terms of surface (SSRR; mm2 ind−1 h−1) and volume (SSRR; mm3 ind−1 h−1) as follows:
where (mm h−1) and (mm h−1) are the surface of sediment reworked between two successive acquisitions associated with a depression or an elevation of the sediment surface, respectively. Here, N is the number of individuals in the Petri dish (N=15).
where (mm3 h−1) and (mm3 h−1) are the volume of sediment reworked between two successive acquisitions associated with a diminution or augmentation of the sediment surface, respectively. Here, N is the number of individuals in the Petri dish (N=15).
Finally, both the surfaces and volumes of reworked sediment were averaged to estimate daily rates, SSRR (mm2 ind−1 d−1) and SSRR (mm3 ind−1 d−1), respectively. These results could thus be compared to those obtained with the SIA method (e.g. Bouchet and Seuront, 2020; Deldicq et al., 2021) and to sediment reworking rates reported in previous studies involving sediment microtopography mapping (e.g. Massé et al., 2019).
2.4 Calculation of the sediment reworking rate with the surface image analysis method
The SIA method requires a regular recording of the displacements of each individual at the sediment surface (Bouchet and Seuront, 2020; Deldicq et al., 2021; Maire et al., 2008). Due to the impossibility of tracking with precision the individuals between the 2 consecutive days of the experiment, the position of each foraminifer was only detected between 24 and 34 h. The coordinates (x, y) within the image were extracted with the image analysis software Fiji (https://imagej.net/software/fiji/, last access: 10 February 2025) using the Manual tracking plugin (Schindelin et al., 2012).
The distance travelled (Dt, mm) in 1 h by a single individual was calculated as follows (Eq. 3):
where (xt, yt) and (xt+1, yt+1) are the coordinates of each individual between two successive pictures.
The volume of sediment reworked between two successive acquisitions (SSRR; mm3 ind−1 h−1) was calculated as follows for each individual (Eq. 4):
where Dt is the distance travelled (mm) by an individual between two successive acquisitions (1 h) and S is the surface of the test (mm2). Since, due to their small size, individuals could not be properly identified during the tracking process, the same average value of the test surface (S) was used for specimens of the same replicate (see Table 1). Note that, when an individual used a pre-existing track, SSRR was considered null during this period of time.
The test surface of each foraminifer (Si, mm2) was calculated as follows (Eq. 5):
where length (mm) and width (mm) were measured with Fiji on the umbilical side of the foraminifer prior to the experiment.
After being deposited at the sediment surface, the foraminifera can be located at three different positions depending on their behaviour: (1) on the surface, (2) at the sediment–water interface (i.e. semi-buried), and (3) buried below the sediment surface. Obviously, these positions have different impacts on the calculation of SSRRV. Accordingly, the surface of the test in contact with the sediment changes with these positions, and, to apply this impact in the calculation, the variable S (i.e. the surface of the test) was weighted by a factor of , , and 1, corresponding to the three possible positions, respectively (Fig. 3).
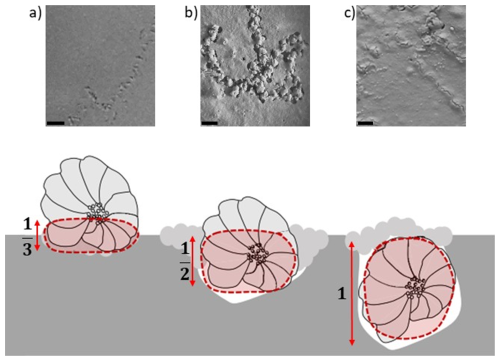
Figure 3Vertical position of foraminifera (a) onto the sediment, (b) at the sediment surface, and (c) buried below the sediment surface and the factor associated to their position in the calculation of surface sediment reworking rate. Scaled bar = 0.2 mm (modified from Deldicq et al., 2021).
The total volume of sediment reworked by each individual in terms of volume (SSRR; mm3 ind−1 d−1) was thus estimated as follows (Eq, 6):
where SSRR (mm3 ind−1 h−1) is the individual surface sediment reworking rate between two successive acquisitions.
3.1 Tracking of Ammonia confertitesta individuals using surface image analysis methods
Among the 45 individuals (15 per replicate) introduced at the beginning of the experiment, only 16 could be tracked at the sediment surface (4, 5, and 7 individuals, respectively, in each replicate, i.e. Petri dish). Data analyses revealed six different behaviours: (i) 10 specimens moved exclusively at the sediment–water interface (individuals 2 and 4 in Fig. 4), (ii) 2 specimens exclusively moved buried just below the sediment surface (individual 1 of the Fig. 4), (iii) 1 specimen moved exclusively onto the sediment, (iv) 1 specimen moved successively onto and below the sediment surface, (v) 1 specimen moved onto the sediment surface and also exhibited a track-following behaviour (i.e. used a track made by another specimen) (individual 3 of the Fig. 4), and (vi) 1 specimen only exhibited a track-following behaviour and was therefore not considered in the estimation of reworking rates (individual 5 of the Fig. 4).
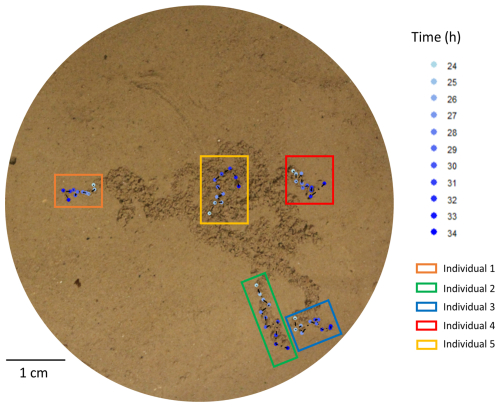
Figure 4Examples of individuals of Ammonia confertitesta tracked during 10 h (from 24 to 34 h following their deposition at the sediment surface) using the surface image analysis method.
During the last 10 h of the experiment (from 24 to 34 h), the SSRRV estimated through the SIA method varied between 0.06±0.04 and 0.09±0.04 mm3 ind−1 h−1, averaging 0.08±0.01 mm3 ind−1 h−1 (Fig. 5).
3.2 Quantification of surface sediment reworking with the 3D sensor method
In the control treatment, there was no modification of the sediment surface microtopography. Conversely, the presence of Ammonia confertitesta induced detectable modifications of the sediment microtopography (Fig. 6).
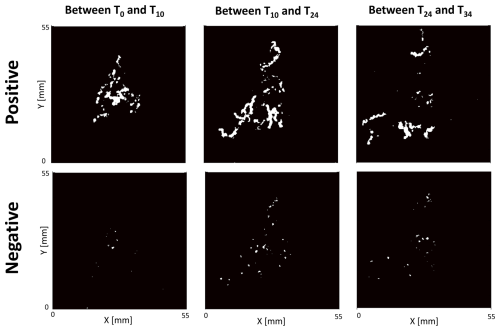
Figure 6Example of surface sediment reworked (in white) by Ammonia confertitesta quantified with a 3D sensor. Only 3 time intervals of one replicate (i.e. one Petri dish filled with an 8 mm thick layer of sediment with 15 individuals) are shown in this figure.
As shown by the four calculated indices, the intensity of the sediment reworking generated by this foraminiferal species at the sediment surface varied through time (Fig. 7). During the first 10 h of the experiment, “Positive” and “Negative” volumes and surfaces of reworked sediment averaged 0.19±0.04, 0.10±0.03, 0.63±0.11, and 0.34±0.11 mm2 ind−1 h−1, respectively. These values were higher than those calculated for the last 10 h of the experiment, where the same indices averaged 0.12±0.03, 0.05±0.02, 0.46±0.10, and 0.18±0.07 mm2 ind−1 h−1, respectively.
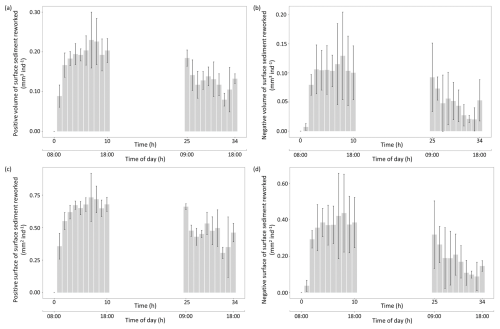
Figure 7Temporal changes in individual sediment reworking rate (SSRRi) generated by Ammonia confertitesta measured with the 3D sensor of the three replicates (the bar represents the mean, and the vertical line represents the SD). Values are expressed in terms of volume (mm3 ind−1) or surface (mm2 ind−1) associated with a net elevation (a, b) or depression (c, d) of the initial sediment surface.
Moreover, the comparison of the four indices revealed that the activity of Ammonia confertitesta during the 34 h experimental runtime mainly induced an elevation of the sediment surface due to particle transport and decompaction (Positive Volume: 3.38±0.40 mm3 ind−1 d−1; Positive Surface: 12.26±1.24 mm2 ind−1 d1) rather than a depression (Negative Volume: 1.14±0.44 mm3 ind−1 d−1; Negative Surface: 4.21±1.30 mm2 ind−1 d−1) (Fig. 8).
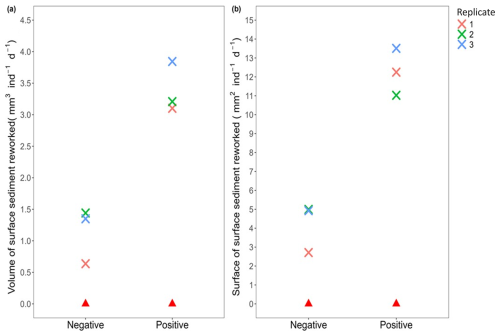
Figure 8Sediment reworking rates calculated in the presence (cross; each colour represents a replicate, i.e. Petri dish) and absence (red triangle) of Ammonia confertitesta: (a) the total volume of sediment excavated (= Negative) and transported onto the initial sediment–water interface (= Positive) (mm3 ind−1 d−1) and (b) the surface of sediment impacted by a depression (= Negative) and an elevation (= Positive) of the initial sediment–water interface (mm2 ind−1 d−1).
3.3 Comparison between the surface sediment reworking rate obtain by 3D sensor method and surface image analysis
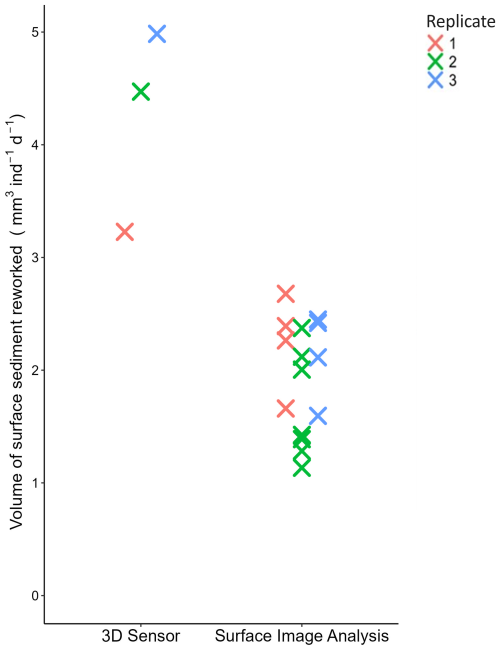
The sediment reworking rate measured at the sediment–water interface (SSRRV) was on average 2 times higher when based on the microtopography mapping with the 3D sensor method (4.23±0.90 mm3 ind−1 d−1) than on the SIA method (1.95±0.50 mm3 ind−1 d−1) (Fig. 9).
Meiofauna, classically defined by a body size ranging between 63 and 1000 µm, are by far the most abundant size class in zoobenthic communities (Coull, 1999). Understanding their contribution to ecosystem functioning, and particularly through sediment bioturbation, is an ongoing and challenging field of research in marine ecology. Although sometimes visible to the naked eye, the minute size of the dominant meiofaunal species, such as foraminifera, nematodes, copepods, or annelids, generates sediment particle transports that are difficult to quantify mainly because of the thin layer of reworked sediment and the challenge of capturing these changes with classical measurement methods developed for macrofaunal species (see Maire et al., 2008).
In consequence, only a few studies have attempted so far to measure particle reworking generated by foraminiferal species at the sediment surface (Bouchet and Seuront, 2020; Deldicq et al., 2021). They all applied the SIA method, which is non-destructive and thus allows successive measurements over relatively long periods of time. However, the SIA method, initially developed to investigate motion and foraging strategy behaviour in meiobenthic organisms (Maire et al., 2016; Bouchet and Seuront, 2020), does not allow the genuine quantification of sediment transport. Indeed, in the present study, following previous works using the SIA method with foraminifera (Bouchet and Seuront, 2020; Deldicq et al., 2021), the surface sediment reworking rate was approximated with (1) the measure of the length of the path travelled over the duration of the experiment by each individual and (2) the average surface of the test of the individuals that were added in the experimental setup. These two metrics were then used to estimate surface sediment reworking rate as a function of the travelled distance and surface of the test. Therefore, it only provided a rough estimation of reworking rates indirectly inferred from the average distance travelled by individuals during a given period of time and their mean body surface. It may further lead to inaccurate quantitative results, especially in foraminifera, since (1) many species are known to build a cyst (i.e. a sedimentary envelope) surrounding their test, which temporarily increases their size and thus the volume of sediment displaced during locomotion events (Heinz et al., 2005), and (2) the volume of reworked sediment tightly depends on the position of individuals at the sediment–water interface (i.e. at the surface, half-buried, completely buried) (Deldicq et al., 2021). Furthermore, specimens moving at the sediment surface are easier to track than those burrowed in it. It is also often challenging to track several individuals efficiently even during relatively short periods of time (e.g. a few hours) due to crossed trajectories or burying activities just below the sediment surface. In the present study, for instance, only one-third of the 45 individuals remained near the sediment interface and could be successfully tracked. This led to some uncertainties on the value of SSRRV obtained with the SIA method.
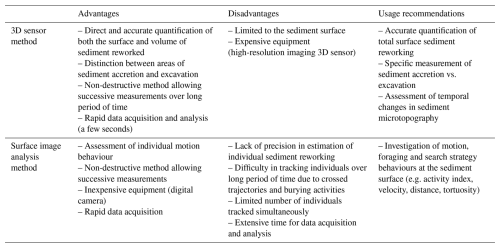
In the present study, the 3D sensor resolution was accurate enough to detect particle transport by benthic foraminifera and to further quantify the sediment reworking rate through the assessment of temporal changes in the sediment surface microtopography. Conversely to SIA, the use of a 3D sensor allows a direct quantification of particle transport, irrespective of the population density, through the assessment of temporal changes in surface microtopography. The high spatial resolution (100 µm for the horizontal dimensions and 35 µm for the vertical dimension) provides an accurate measurement of surface sediment reworking rates, an important mode of bioturbation induced by foraminiferal species living at the benthic interface (Deldicq et al., 2021). Therefore, in the present study, the 3D sensor method allowed the distinction between areas of sediment accretion and excavation generated by benthic foraminiferal individuals (and the calculation of specific “Positive” vs. “Negative” surfaces and volumes), which may have contrasting effects on the physical and biogeochemical dynamics of the benthic interface (Massé et al., 2019; Røy et al., 2002). Another advantage of this method is that the time required to capture the microtopography of the sediment surface is almost instantaneous (i.e. less than 1 s for a 65 cm2 area) and can thus provide accurate measurements of sediment reworking with a high temporal resolution, which is particularly valuable when investigating rapid changes in bioturbation modes and rates due to intermittent behavioural alteration by biotic (e.g. presence of a predator) or abiotic (e.g. food availability) factors. A disadvantage of the 3D detector compared to SIA is that, in the present study, it was not possible to keep track of the motion behaviour of each specimen with the 3D detector. Motion behaviour is also a key parameter to assess, as the intensity and the mode of sediment reworking are constrained by the way an individual and a species are being active at the sediment surface and in the sediment matrix (Deldicq et al., 2020).
Table 2Summary of the advantages, disadvantages, and usage recommendations for the 3D sensor and surface image analysis methods.
From an ecological standpoint, our results showed that Ammonia confertitesta can generate an intense horizontal sediment reworking, averaging 4.23±0.90 mm3 ind−1 d−1. Hence, since A. confertitesta is often found at very high densities, up to 249 800 ind m−2 (Pavard et al., 2023), its reworking activity may likely contribute to the reworking and homogenization of the sediment surface (including the spatial distribution of organic matter) at rates comparable to some macrofaunal at natural density (e.g. on average 45.21 cm3 d−1 for Melinna palmata, an abundant polychaeta on the Atlantic French coast (Massé et al., 2019), and 1056.65 cm3 d−1 for Ammonia confertitesta). Interestingly, it may also thereby indirectly play a role in stimulating vertical particle mixing by increasing the feeding area of surface deposit feeders (Wheatcroft et al., 1990). For example, during the 34 h of the experiment, it clearly appeared that the motion behaviour of Ammonia confertitesta mainly generated an elevation of the initial sediment–water interface, as sediment accretion was about 3-fold higher than excavation. Such a sediment reworking mode has already been reported for several epibenthic macrofaunal species (e.g. tentaculate annelids and gastropod molluscs) and typically results in the decompaction of the surficial sediment layer, which in turn may strongly enhance its erodibility and pore-water exchanges between the benthic and pelagic compartments (Massé et al., 2019; Orvain et al., 2004). Indeed, it was reported that the pseudopodial activity and the motion behaviour of the benthic foraminifera Ammonia cf. aomoriensis decreased sediment stability, enhancing the bentho-pelagic coupling (Cedhagen et al., 2021). In addition, the 3D method can be used in combination with other biogeochemical analyses (e.g. O2 or nutrient flux measurements across the sediment surface), to provide a more exhaustive understanding of the foraminiferal role in bioturbation processes.
The comparison of the two methods confirmed our hypothesis that microtopography mapping with the 3D detector provides a sound quantification of particle transport by meiofaunal organisms which are likely more accurate than SIA due to calculation approximations (Tables 1 and 2 and the abovementioned limitations). Furthermore, even though the time of data acquisition is the same between the two methods (i.e. less than 1 s for each acquisition), the data processing required by the SIA method is time consuming, as (1) it involves the measuring of each individual before the experiment, (2) individual tracking can hardly be fully automatized due to the minute size of foraminiferal individual and the presence of sediment, and (3) the estimation of the volume of sediment displaced between two pictures has to be weighted according to the vertical position (i.e. at the surface, half-burrowed, totally burrowed; Fig. 3) of each individual at the sediment surface for all the different acquisitions. However, SIA is more adapted than the 3D detector to study specific questions related to behavioural traits (e.g. activity index, velocity, distance, tortuosity) at the individual scale.
This study demonstrates that sediment microtopography mapping using a 3D sensor is a powerful method for accurately quantifying particle transport processes generated by benthic meiofaunal species at the sediment–water interface. It provides more reliable estimates of sediment reworking rates compared to the most commonly used method, which is based on sediment image analysis. The implementation of the 3D sensor in bioturbation studies will certainly enhance our ability to assess and understand the role of benthic foraminifera, in particular, along with other meiofaunal species (e.g. nematodes, copepods, and oligochaetes) in soft-sediment ecosystem functioning. Furthermore, it holds significant potential to complement the particle tracer method, which is suitable for investigating the vertical (1D) transport of surficial particles to deeper layers (Deldicq et al., 2023; Maire et al., 2008) but less efficient for assessing horizontal transport occurring at the sediment surface.
The data are available on Zenodo: https://doi.org/10.5281/zenodo.17185302 (Doutrelant et al., 2025).
The supplement related to this article is available online at https://doi.org/10.5194/jm-44-401-2025-supplement.
MD: conceptualization, investigation, methodology, formal analysis, visualization, writing (original draft). ARR: conceptualization, data curation, investigation, methodology, formal analysis, writing (original draft). AC: investigation, writing (review and editing). VMPB: conceptualization, methodology, funding acquisition, formal analysis, supervision, writing (review and editing). OM: conceptualization, investigation, funding acquisition, methodology, formal analysis, supervision, writing (review and editing).
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
This article is part of the special issue “Advances and challenges in modern and benthic foraminifera research: a special issue dedicated to Professor John Murray”. It is not associated with a conference.
The authors are grateful to Hugues Blanchet and Vaéa Bujan for performing the sampling at the Phare of Richard, to Claire Pollet-Calderini for suggesting some modification on the MATLAB analysis, and to Julien Richirt for his suggestions and comments on an earlier version of the article.
This work was supported by the COFFEE project funded by the French National program EC2CO (Ecosphère Continentale et Côtier, CNRS-INSU). Manon Doutrelant received a PhD fellowship from the Region Hauts-de-France and the University of Lille.
This paper was edited by Babette Hoogakker and reviewed by Brett Metcalfe and one anonymous referee.
Aller, R. C.: Sedimentary Diagenesis, Depositional Environments, and Benthic Fluxes, in: Treatise on Geochemistry, Elsevier, 293–334, https://doi.org/10.1016/B978-0-08-095975-7.00611-2, 2014.
Bernard, G., Gammal, J., Järnström, M., Norkko, J., and Norkko, A.: Quantifying bioturbation across coastal seascapes: Habitat characteristics modify effects of macrofaunal communities, J. Sea Res., 152, 101766, https://doi.org/10.1016/j.seares.2019.101766, 2019.
Bernstein, B. B., Hessler, R. R., Smith, R., and Jumars, P. A.: Spatial dispersion of benthic Foraminifera in the abyssal central North Pacific 1, Limnol. Oceanogr., 23, 401–416, https://doi.org/10.4319/lo.1978.23.3.0401, 1978.
Bonaglia, S., Nascimento, F. J. A., Bartoli, M., Klawonn, I., and Brüchert, V.: Meiofauna increases bacterial denitrification in marine sediments, Nat. Commun., 5, 5133, https://doi.org/10.1038/ncomms6133, 2014.
Bonaglia, S., Hedberg, J., Marzocchi, U., Iburg, S., Glud, R. N., and Nascimento, F. J. A.: Meiofauna improve oxygenation and accelerate sulfide removal in the seasonally hypoxic seabed, Mar. Environ. Res., 159, 104968, https://doi.org/10.1016/j.marenvres.2020.104968, 2020.
Bouchet, V. M. P. and Seuront, L.: Strength may lie in numbers: Intertidal Foraminifera non-negligible contribution to surface sediment reworking, Open J. Mar. Sci., 10, 131–140, https://doi.org/10.4236/ojms.2020.103010, 2020.
Buffoni, G., Delfanti, R., and Papucci, C.: Accumulation rates and mixing processes in near-surface North Atlantic sediments: Evidence from C-14 and Pu-239,240 downcore profiles, Mar. Geol., 109, 159–170, https://doi.org/10.1016/0025-3227(92)90226-8, 1992.
Cedhagen, T., Mamuaja, J. M., and Lund-Hansen, L. C.: The sediment reworking foraminiferan Ammonia cf. aomoriensis is a sediment destabilizer: Insights from an experiment with artificial removal of the pseudopods, Reg. Stud. Mar. Sc., 45, 101814, https://doi.org/10.1016/j.rsma.2021.101814, 2021.
Cesbron, F., Geslin, E., Jorissen, F. J., Delgard, M. L., Charrieau, L., Deflandre, B., Jézéquel, D., Anschutz, P., and Metzger, E.: Vertical distribution and respiration rates of benthic Foraminifera: Contribution to aerobic remineralization in intertidal mudflats covered by Zostera noltei meadows, Estuar. Coast. Shelf Sci., 179, 23–38, https://doi.org/10.1016/j.ecss.2015.12.005, 2016.
Chandler, G. T.: Foraminifera may structure meiobenthic communities, Oecologia, 81, 354–360, https://doi.org/10.1007/BF00377083, 1989.
Coull, B. C.: Role of meiofauna in estuarine soft-bottom habitats, Aust. J. Ecol., 24, 327–343, https://doi.org/10.1046/j.1442-9993.1999.00979.x, 1999.
Dairain, A., Maire, O., Meynard, G., Richard, A., Rodolfo-Damiano, T., and Orvain, F.: Sediment stability: can we disentangle the effect of bioturbating species on sediment erodibility from their impact on sediment roughness?, Mar. Environ. Res., 162, 105147, https://doi.org/10.1016/j.marenvres.2020.105147, 2020.
De Borger, E., Tiano, J., Braeckman, U., Ysebaert, T., and Soetaert, K.: Biological and biogeochemical methods for estimating bioirrigation: a case study in the Oosterschelde estuary, Biogeosciences, 17, 1701–1715, https://doi.org/10.5194/bg-17-1701-2020, 2020.
Deldicq, N., Seuront, L., Langlet, D., and Bouchet, V.: Assessing behavioural traits of benthic Foraminifera: Implications for sediment mixing, Mar. Ecol.-Prog. Ser., 643, 21–31, https://doi.org/10.3354/meps13334, 2020.
Deldicq, N., Seuront, L., and Bouchet, V. M. P.: Inter-specific and inter-individual trait variability matter in surface sediment reworking rates of intertidal benthic Foraminifera, Mar. Biol., 168, 101, https://doi.org/10.1007/s00227-021-03908-w, 2021.
Deldicq, N., Mermillod-Blondin, F., and Bouchet, V. M. P.: Sediment reworking of intertidal sediments by the benthic Foraminifera Haynesina germanica: the importance of motion behaviour and densities, P. Roy. Soc. B, 290, 20230193, https://doi.org/10.1098/rspb.2023.0193, 2023.
Doutrelant, M., Romero Ramirez, A., Ciutat, A., Bouchet, V., and Maire, O.: Quantitative measurement of benthic foraminifera sediment reworking using a 3-dimensional sensor, Zenodo [data set], https://doi.org/10.5281/zenodo.17185302, 2025.
Fouet, M., Daviray, M., Geslin, E., Metzger, E., and Jorissen, F.: Foraminiferal test dissolution reveals severe sediment acidification in estuarine mudflats: new perspectives for present and historical assessment, Comptes Rendus Géoscience, 356, 83–96, https://doi.org/10.5802/crgeos.269, 2024a.
Fouet, M. P. A., Schweizer, M., Singer, D., Richirt, J., Quinchard, S., and Jorissen, F. J.: Unravelling the distribution of three Ammonia species (Foraminifera, Rhizaria) in French Atlantic Coast estuaries using morphological and metabarcoding approaches, Mar. Micropaleontol., 188, 102353, https://doi.org/10.1016/j.marmicro.2024.102353, 2024b
Gautreau, E., Volatier, L., Nogaro, G., Gouze, E., Marmonier, P., and Mermillod-Blondin, F.: Interactions between microbial activity and bioturbation modes of benthic invertebrates determine nutrient releases from reservoir sediments, Freshwater Biol., 68, 245–259, https://doi.org/10.1111/fwb.14021, 2023.
Gross, O.: Sediment interactions of Foraminifera: Implications for food degradation and bioturbation processes, J. Foramin. Res., 32, 414–424, https://doi.org/10.2113/0320414, 2002.
Heinz, P., Geslin, E., and Hemleben, C.: Laboratory observations of benthic foraminiferal cysts, Mar. Biol. Res., 1, 149–159, https://doi.org/10.1080/17451000510019114, 2005.
Henderson, G. M., Lindsay, F. N., and Slowey, N. C.: Variation in bioturbation with water depth on marine slopes: A study on the Little Bahamas Bank, Mar. Geol., 160, 105–118, https://doi.org/10.1016/S0025-3227(99)00018-3, 1999.
Kim, K. H. and Burnett, W. C.: Accumulation and biological mixing of Peru margin sediments, Mar. Geol., 80, 181–194, https://doi.org/10.1016/0025-3227(88)90089-8, 1988.
Kristensen, E., Penha-Lopes, G., Delefosse, M., Valdemarsen, T., Quintana, C., and Banta, G.: What is bioturbation? The need for a precise definition for fauna in aquatic sciences, Mar. Ecol.-Prog. Ser., 446, 285–302, https://doi.org/10.3354/meps09506, 2012.
Langlet, D., Mermillod-Blondin, F., Deldicq, N., Bauville, A., Duong, G., Konecny, L., Hugoni, M., Denis, L., and Bouchet, V. M. P.: Single-celled bioturbators: Benthic Foraminifera mediate oxygen penetration and prokaryotic diversity in intertidal sediment, Biogeosciences, 20, 4875–4891, https://doi.org/10.5194/bg-20-4875-2023, 2023.
Maire, O., Duchêne, J., Bigot, L., and Grémare, A.: Linking feeding activity and sediment reworking in the deposit-feeding bivalve Abra ovata with image analysis, laser telemetry, and luminophore tracers, Mar. Ecol.-Prog. Ser., 351, 139–150, https://doi.org/10.3354/meps07147, 2007.
Maire, O., Lecroart, P., Meysman, F., Rosenberg, R., Duchêne, J., and Grémare, A.: Quantification of sediment reworking rates in bioturbation research: a review, Aquat. Biol., 2, 219–238, https://doi.org/10.3354/ab00053, 2008.
Maire, O., Barras, C., Gestin, T., Nardelli, M., Romero-Ramirez, A., Duchêne, J., and Geslin, E.: How does macrofaunal bioturbation influence the vertical distribution of living benthic Foraminifera?, Mar. Ecol.-Prog. Ser., 561, 83–97, https://doi.org/10.3354/meps11929, 2016.
Massé, C., Garabetian, F., Deflandre, B., Maire, O., Costes, L., Mesmer-Dudons, N., Duchêne, J.-C., Bernard, G., Grémare, A., and Ciutat, A.: Feeding ethology and surface sediment reworking by the ampharetid polychaete Melinna palmata Grube, 1870: Effects on sediment characteristics and aerobic bacterial community composition, J. Exp. Mar. Biol. Ecol., 512, 63–77, https://doi.org/10.1016/j.jembe.2018.12.009, 2019.
Meadows, P. S. and Tufail, A.: Bioturbation, microbial activity and sediment properties in an estuarine ecosystem, P. Roy. Soc. B, 90, 129–142, https://doi.org/10.1017/S0269727000004930, 1986.
Mermillod-Blondin, F., Rosenberg, R., François-Carcaillet, F., Norling, K., and Mauclaire, L.: Influence of bioturbation by three benthic infaunal species on microbial communities and biogeochemical processes in marine sediment, Aquat. Microb. Ecol., 36, 271–284, https://doi.org/10.3354/ame036271, 2004.
Murray, J. W.: Biodiversity of living benthic foraminifera: How many species are there?, Mar. Micropaleontol., 64, 163–176, https://doi.org/10.1016/j.marmicro.2007.04.002, 2007.
Nelson, C. H., Johnson, K. R., and Barber, J. H.: Gray Whale and Walrus Feeding Excavation on the Bering Shelf, Alaska, J. Sediment. Petrol., 57, 419–430, https://doi.org/10.1306/212F8B4D-2B24-11D7-8648000102C1865D, 1987.
Orvain, F., Sauriau, P., Sygut, A., Joassard, L., and Le Hir, P.: Interacting effects of Hydrobia ulvae bioturbation and microphytobenthos on the erodibility of mudflat sediments, Mar. Ecol.-Prog. Ser., 278, 205–223, https://doi.org/10.3354/meps278205, 2004.
Pavard, J.-C., Richirt, J., Seuront, L., Blanchet, H., Fouet, M. P. A., Humbert, S., Gouillieux, B., Duong, G., and Bouchet, V. M. P.: The great shift: The non-indigenous species Ammonia confertitesta (Foraminifera, Rhizaria) outcompetes indigenous Ammonia species in the Gironde estuary (France), Estuar. Coast. Shelf Sci., 289, 108378, https://doi.org/10.1016/j.ecss.2023.108378, 2023.
Queirós, A. M., Birchenough, S. N. R., Bremner, J., Godbold, J. A., Parker, R. E., Romero-Ramirez, A., Reiss, H., Solan, M., Somerfield, P. J., Van Colen, C., Van Hoey, G., and Widdicombe, S.: A bioturbation classification of European marine infaunal invertebrates, Ecol. Evol., 3, 3958–3985, https://doi.org/10.1002/ece3.769, 2013.
Richard, A., Orvain, F., Morelle, J., Romero-Ramirez, A., Bernard, G., Paulin-Henricksson, S., Cordier, M.-A., Montaudouin, X. D., and Maire, O.: Impact of Sediment Bioturbation on Microphytobenthic Primary Producers: Importance of Macrobenthic Functional Traits, Ecosystems, 26, 1077–1094, https://doi.org/10.1007/s10021-022-00817-x, 2023.
Røy, H., Hüttel, M., and Jørgensen, B. B.: The role of small-scale sediment topography for oxygen flux across the diffusive boundary layer, Limnol. Oceanogr., 47, 837–847, https://doi.org/10.4319/lo.2002.47.3.0837, 2002.
Røy, H., Huettel, M., and Jørgensen, B. B.: The influence of topography on the functional exchange surface of marine soft sediments, assessed from sediment topography measured in situ, Limnol. Oceanogr., 50, 106–112, https://doi.org/10.4319/lo.2005.50.1.0106, 2005.
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J.-Y., White, D. J., Hartenstein, V., Eliceiri, K., Tomancak, P., and Cardona, A.: Fiji: an open-source platform for biological-image analysis, Nat. Meth., 9, 676–682, https://doi.org/10.1038/nmeth.2019, 2012.
Schratzberger, M. and Ingels, J.: Meiofauna matters: The roles of meiofauna in benthic ecosystems, J. Exp. Mar. Biol. Ecol., 502, 12–25, https://doi.org/10.1016/j.jembe.2017.01.007, 2018.
Severin, K. P., Culver, S. J., and Blanpied, C.: Burrows and trails produced by Quinqueloculina impressa Reuss, a benthic foraminifer, in fine-grained sediment, Sedimentology, 29, 897–901, https://doi.org/10.1111/j.1365-3091.1982.tb00093.x, 1982.
Shen, H., Jiang, G., Wan, X., Li, H., Qiao, Y., Thrush, S., and He, P.: Response of the microbial community to bioturbation by benthic macrofauna on intertidal flats, J. Exp. Mar. Biol. Ecol., 488, 44–51, https://doi.org/10.1016/j.jembe.2016.12.010, 2017.
Shull, D. H., Benoit, J. M., Wojcik, C., and Senning, J. R.: Infaunal burrow ventilation and pore-water transport in muddy sediments, Estuar. Coast. Shelf Sci., 83, 277–286, https://doi.org/10.1016/j.ecss.2009.04.005, 2009.
Solan, M., Batty, P., Bulling, M., and Godbold, J.: How biodiversity affects ecosystem processes: implications for ecological revolutions and benthic ecosystem function, Aquat. Biol., 2, 289–301, https://doi.org/10.3354/ab00058, 2008.
Wheatcroft, R. A., Jumars, P. A., Smith, C. R., and Nowell, A. R. M.: A mechanistic view of the particulate biodiffusion coefficient: Step lengths, rest periods and transport directions, J. Mar. Res., 48, 177–207, https://doi.org/10.1357/002224090784984560, 1990.
Zeppilli, D., Leduc, D., Fontanier, C., Fontaneto, D., Fuchs, S., Gooday, A. J., Goineau, A., Ingels, J., Ivanenko, V. N., Kristensen, R. M., Neves, R. C., Sanchez, N., Sandulli, R., Sarrazin, J., Sørensen, M. V., Tasiemski, A., Vanreusel, A., Autret, M., Bourdonnay, L., Claireaux, M., Coquillé, V., De Wever, L., Rachel, D., Marchant, J., Toomey, L., and Fernandes, D.: Characteristics of meiofauna in extreme marine ecosystems: a review, Mar. Biodiv., 48, 35–71, https://doi.org/10.1007/s12526-017-0815-z, 2018.