the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Revisiting Early Jurassic Biscutaceae: Similiscutum giganteum sp. nov.
Samuel Mailliot
Emanuela Mattioli
Micaela Chaumeil Rodríguez
Bernard Pittet
A large, broadly elliptical coccolith of the genus Similiscutum (Biscutaceae) was observed in sediments dated from the Lower Jurassic (upper Pliensbachian to Toarcian) coming from different localities of western Tethys, namely Portugal (Lusitanian Basin), France (Causses and Paris basins) and Spain (Subbetic area). This form is quite easy to find in the Toarcian GSSP (Global Stratotype Section and Point) of Peniche (Portugal), where the holotype has been described. More than 100 specimens of Similiscutum were digitally captured using a CCD camera, including this large form and two other related species, Similiscutum finchii and Similiscutum novum. The length and width of the coccoliths and the length and width of their central area were measured, and biometric analyses were performed. Results show that this large morphotype of Similiscutum is well characterized and easily differentiable by its size and morphology from the species S. finchii and S. novum, which are characterized by a similar extinction pattern in optical-microscope crossed polars . On the basis of combined differences in size and in central-area shape and structure, Similiscutum giganteum sp. nov. is introduced here. (Plant Fossil Names Registry no.: PFN003067; Act LSID: urn:lsid:plantfossilnames.org:act:3067).
- Article
(7535 KB) - Full-text XML
-
Supplement
(375 KB) - BibTeX
- EndNote
The Biscutaceae are the oldest known placolith coccoliths, appearing at the very base of the Pliensbachian (Bown, 1987a, b; de Kaenel and Bergen, 1993; Mattioli and Erba, 1999; Fraguas et al., 2015, 2018; Peti et al., 2017; Ferreira et al., 2019). During the Pliensbachian and the early Toarcian, this family underwent an important diversification and became a major component of Lower Jurassic calcareous nannofossil assemblages (Bown, 1987a; Mattioli and Erba, 1999; Wiggan et al., 2018).
Lower Jurassic coccoliths belonging to the family Biscutaceae Black, 1971, emend. Bown, 1987a, have been relatively well known since the publication of taxonomic works by Bown (1987a) and de Kaenel and Bergen (1993), where the genus Similiscutum was first introduced, and by Mattioli et al. (2004), where the differentiation between Lower Jurassic species belonging to the genera Biscutum and Similiscutum was quantitatively assessed using biometrics. However, a large Similiscutum species, morphologically close to Similiscutum finchii from an ultrastructural and optical point of view, seems to stay undescribed yet. This morphotype was observed in several sections of western Tethys, namely in Portugal (Peniche section; Mailliot, 2006; Ferreira et al., 2019), France (Causses and distal margin of Armorican Massif; Mailliot, 2006; Menini et al., 2019) and Spain (Subbetic area; Reolid et al., 2014). In the literature, Bown (1987a) already mentioned very large specimens of Similiscutum finchii recorded in an Argentinian section (Picún Leufú). Subsequently, other papers figured this form to be a representative of Biscutum (= Similiscutum) finchii (e.g. Perilli and Comas-Rengifo, 2002; Perilli and Duarte, 2006; Fraguas et al., 2008; see list of synonymies for further details).
De Kaenel and Bergen (1993) separated the genus Similscutum from Biscutum and described several new species in Similiscutum; this allowed an improved biochronological subdivision of the Jurassic period. Since then, the first occurrence of placoliths of Similiscutum have been used to define the lowermost nannofossil zone of the Pliensbachian stage (Bown and Cooper, 1998; Mattioli and Erba; 1999; Fraguas et al., 2015, 2018; Peti et al., 2017; Ferreira et al., 2019). This event is now used in the Geologic Time Scale (Hesselbo et al., 2020).
Using biometric analysis, this study aims to quantitatively assess the morphology of the large form of Similiscutum and to differentiate it from the other related Similiscutum species, such as S. finchii and S. novum. Given its distinctive morphology and its stratigraphic range restricted to upper Pliensbachian and Toarcian strata (Ferreira et al., 2019), the potential stratigraphic use of this new species has to be explored.
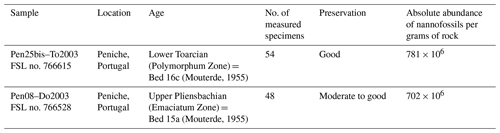
Two rock samples characterized by high absolute abundances of nannofossils and a fairly good coccolith preservation were selected around the Pliensbachian–Toarcian boundary of Peniche (Ponta do Trovão section) in Portugal (Table 1), where the new Similiscutum morphotype is relatively easily recovered compared to other samples or localities. In these two samples, images of a minimum of 15 coccoliths were digitally captured for each of three species – Similiscutum finchii, S. novum and a large form of Similiscutum – in both cross-polarized light and parallel polars. Image acquisition was made using a CCD video camera (Sony XC-77CE) on an “Axioskop 40” Zeiss microscope, using the software “ITI camera configurator” (version 3.3.0.0).
Table 1Stratigraphic information of the studied samples. Repository: Collections de Géologie de Lyon.
The terminology used in this study follows the guidelines of Young et al. (1997). For each specimen, four simple parameters were analysed: the length and width of the coccoliths were measured on parallel polar images, where the outline is more easily detected; the length and width of the coccolith central area were measured on polarized-light images. Measurements were made using the software “Scion Image” (beta version 4.0.2), which is a modified Windows equivalent of the “NIH Image” technology developed for Macintosh. The accuracy of measurements was estimated by multiple-scale calibration (conversion of pixels into micrometres using “Scion Image”) and by multiple dimensional measurements on the same specimen for the four considered parameters. The scale is 1 µm = 9.34±0.02 pixels; the accuracy of scale calibration is ±0.002 µm. Mean error on dimensional measurements is ±0.088 µm. By adding these two types of error, the accuracy of dimensional measurements is ±0.09 µm, which is very close to the error of ±0.1 µm published by Young et al. (1996) for similar biometric methods.
At first, mixture analysis was applied to coccolith length and width of the entire population of measured specimens using the PAST 3.01 software (Hammer et al., 2001). The data were then treated with the software “StatView” (version 5.0). Simple parameters were considered for the morphologic description of the three species, such as the coccolith and central-area dimensions (length and width), the relative proportion of the central area with respect to the coccolith size (calculated as the ratios “central-area length over coccolith length” and “central-area width over coccolith width”), and the coccolith ellipticity (calculated as the ratio “coccolith length over coccolith width”). The frequency distribution of these parameters was compared between the considered species in order to test if the large form of Similiscutum can be significantly differentiated by its size and shape from S. finchii and S. novum. According to the work of Mattioli et al. (2004), a 10th–90th percentile interval was chosen to distinguish between a pair of species by their dimensions. This means that two species can be differentiated by means of one of the considered parameters if the frequency distributions of such parameter are different for more than 80 % of the populations (Mattioli et al., 2004, see Fig. 5). In order to test if the average of measured parameters is significantly different between two given species, the ANOVA (ANalysis Of VAriance) post-hoc Bonferroni–Dunn test was applied. This test evaluates the probability (p) of having the same average for a given parameter for a pair of species. In this study, for a significance level at 5 % in a Bonferroni–Dunn test, the significant level of differences between the average values of a pair of species is p<0.0167. The new measurements acquired in this paper were then compared to published biometric data of Lower Jurassic species of Biscutaceae (Mattioli et al., 2004).
3.1 Mixture analysis
A comparison of one- and two-group models was performed using the whole dataset for coccolith length and width by comparing their associated Akaike information criterion (AIC; Akaike, 1973) according to the statistical procedure developed by Favre et al. (2008) that allows the optimized separation of two sets of normally distributed values. This method is thus based on two a priori-defined sets of values – namely, one with the smallest mean and another with the largest mean – obtained for a given, normally distributed variable (Suchéras-Marx et al., 2010). The number of size classes or bins was chosen in order to obtain each bin at 0.5 µm. The results of mixture analysis show two sets of samples with a mode at 4.0–5.0 and 7.5–8.5 µm for coccolith length and a mode at 3.5–4.5 and 6.5–7.5 µm for coccolith width (Fig. 1). According to mixture analysis, the dataset has a bimodal nature with a larger morphotype (herein named Similiscutum giganteum sp. nov.; Plate 1) and two smaller morphotypes, namely S. finchii and S. novum (Plate 2). In the following, we present boxplot analysis of coccolith geometry separately for the three species.
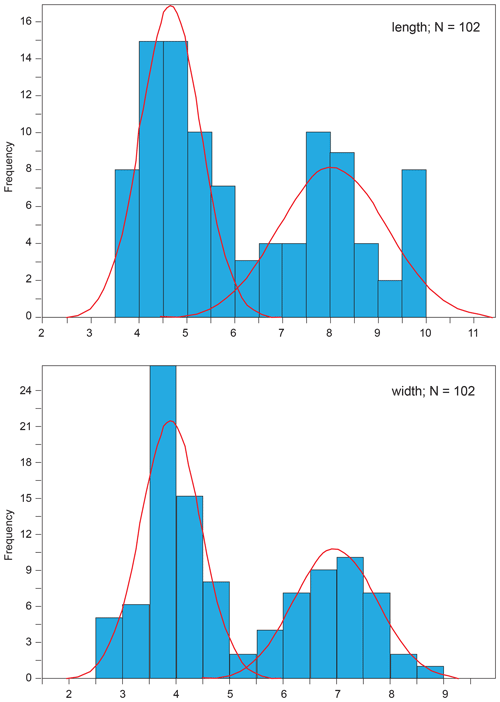
Figure 1Mixture analysis applied to the length and width of all the analysed coccoliths using PAST 3.01 software (Hammer et al., 2001). The lowest Akaike information criterion (AIC, Akaike, 1973) is obtained with two populations with modes at 4.0–5.0 and 7.5–8.5 µm for coccolith length and at 3.5–4.5 and 6.5–7.5 µm for coccolith width. The number of size classes or bins was chosen in order to obtain each bin at 0.5 µm.
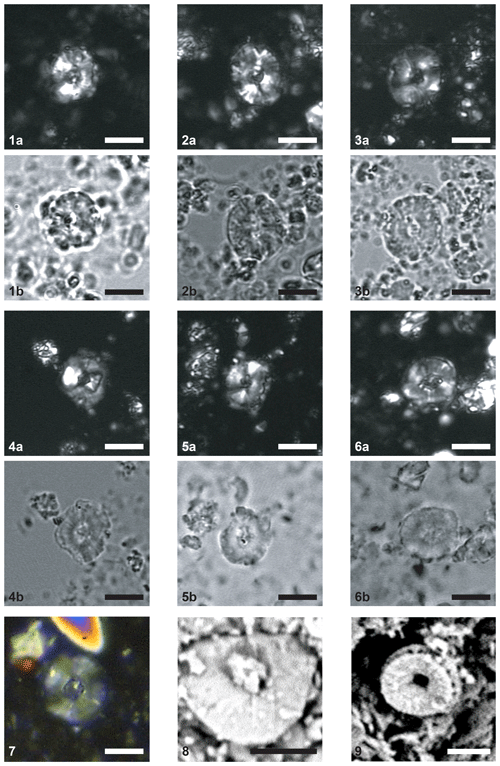
Plate 1Scale bar = 5 µm. CPs indicates crossed polars; PPs indicates parallel polars; SEM indicates scanning electron microscope. (1–6) Similiscutum giganteum sp. nov. (1a) CPs and (1b) PPs, sample PEN08-Do2003 (Emaciatum Zone), holotype. (2a) CPs and (2b) PPs, sample PEN08-Do2003 (Emaciatum Zone). (3a) CPs and (3b) PPs, sample PEN08-Do2003 (Emaciatum Zone). (4a) CPs and (4b) PPs, sample PEN25bis-To2003 (Polymorphum Zone). (5a) CPs and (5b) PPs, sample PEN25bis-To2003 (Polymorphum Zone). (6a) CPs and (6b) PPs, sample PEN25bis-To2003 (Polymorphum Zone). (7) CPs, sample EMII-7 (Toarcian). (8) SEM, La Cerradura section (Spain), sample CE 34 (Polymorphum Zone). (9) SEM, Borehole FR-210-078, Lorraine Sub-Basin, S Luxembourg, sample K004542 (Falciferum Zone).
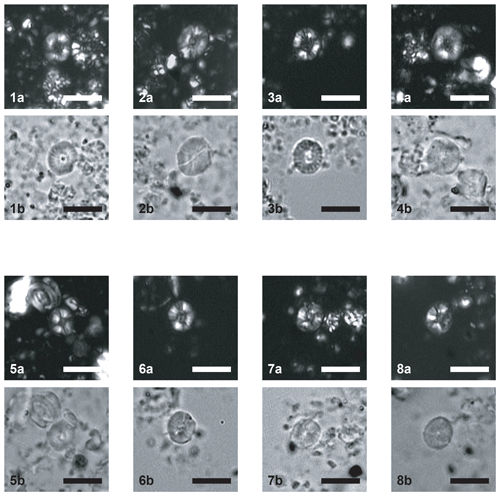
Plate 2Scale bar = 5 µm. CPs indicates crossed polars; PPs indicates parallel polars. (1–4) Similiscutum finchii. (1a) CPs and (1b) PPs, sample PEN08-Do2003 (Emaciatum Zone). (2a) CPs and (2b) PPs, sample PEN08-Do2003 (Emaciatum Zone). (3a) CPs and (3b) PPs, sample PEN08-Do2003 (Emaciatum Zone). (4a) CPs and (4b) PPs, sample PEN25bis-To2003 (Polymorphum Zone). (5–8) Similiscutum novum. (5a) CPs and (5b) PPs, sample PEN25bis-To2003 (Polymorphum Zone). (6a) CPs and (6b) PPs, sample PEN25bis-To2003 (Polymorphum Zone). (7a) CPs and (7b) PPs, sample PEN25bis-To2003 (Polymorphum Zone). (8a) CPs and (8b) PPs, sample PEN25bis-To2003 (Polymorphum Zone).
3.2 Coccolith and central-area dimensions
The frequency distribution of coccolith length and width (Fig. 2a and c) for the three analysed species shows that the large morphotype of Similiscutum, namely S. giganteum sp. nov., can be easily differentiated from S. finchii and S. novum on the basis of the analysed parameters. However, the boxplot displays important overlapping between S. giganteum and S. finchii and between S. finchii and S. novum when taking into account the central-area length and width, which shows a continuum in the size variation (Fig. 2b and d). Based on the Bonferroni–Dunn test, significant differences in the mean sizes between the considered species, both for coccolith and central-area measurements, can be assessed (Table S1 in the Supplement).
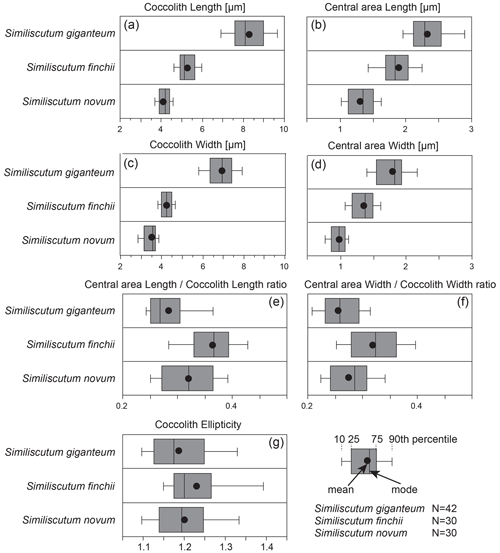
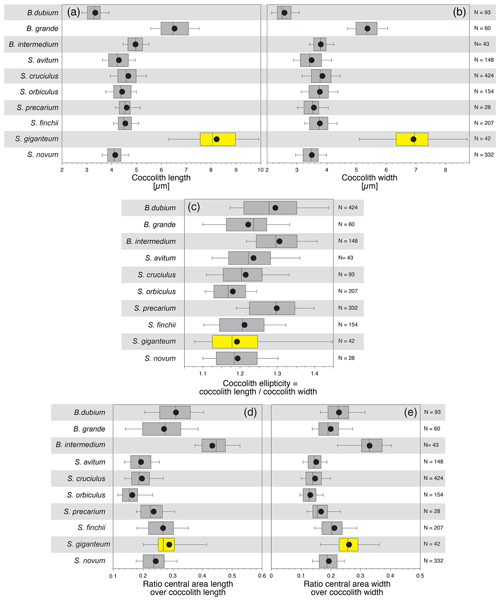
Figure 2Frequency distribution of coccolith length (a) and width (c) and of central-area length (b) and width (d) of the ratios “central-area length over coccolith length” (e) and “central-area width over coccolith width” (f), along with coccolith ellipticity (g) for the three analysed species. N is the number of measured specimens.
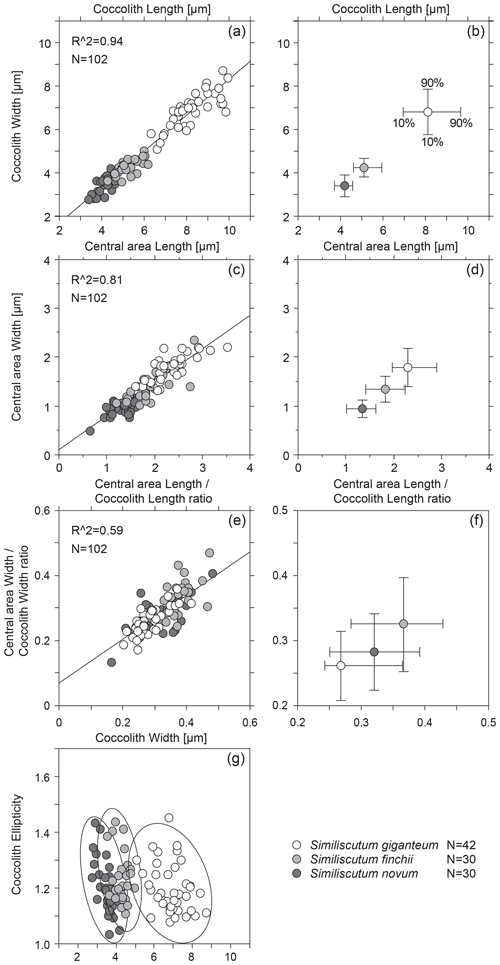
Figure 3Bivariate plot of coccolith length and width (a) and of central-area length and width (c). The calculated R2 is quite high. Mean values with the 10th–90th percentiles of frequency distribution for coccolith length and width (b) and for central-area length and width (d) for the three analysed species. Bivariate plot of the ratios “central-area length over coccolith length” and “central-area width over coccolith width” (e) and mean values with the 10th–90th percentiles of frequency distribution for these two ratios (f) for the three analysed species. Coccolith ellipticity versus coccolith width (g). N is the number of measured specimens.
The bivariate plot of coccolith length and width and of central-area length and width (Fig. 3a and c) shows that there is a strong covariance between the observed parameters. The mean values with the 10th–90th percentiles of frequency distribution for coccolith length and width for the three analysed species (Fig. 3b) allow an easy differentiation of S. giganteum sp. nov. from the cluster composed of S. finchii and S. novum. However, the bivariate plots of the means for central-area length and width show a continuum in size distribution passing from S. novum to S. giganteum (Fig. 3d).
3.3 Coccolith geometry
Simple parameters for coccolith geometry, such as the relative proportion of the central area with respect to the coccolith size, are evaluated as the ratios “central-area length over coccolith length” and “central-area width over coccolith width” (Fig. 2e and f; Table S2). The frequency distribution of these two ratios shows a strong overlap for the three analysed species, but the dimensions of the central area relative to the coccolith dimensions of S. giganteum sp. nov. are comprised in the lower part of the represented values. There are significant differences in the mean sizes between the considered species for the two ratios, except for S. giganteum compared to S. novum (p>0.0167), which both possess a relatively smaller central area than S. finchii (Table S1).
The bivariate plots of the three analysed species (Fig. 3e and f) indicate a fairly good covariance between the ratios “central-area length over coccolith length” and “central-area width over coccolith width”, but the mean central-area dimensions with respect to the coccolith dimensions display a strong overlap between the three species of Similiscutum.
Also, the coccolith ellipticity was calculated as the ratio “coccolith length over coccolith width” (Fig. 2g). The frequency distribution of coccolith ellipticity is quite similar for the three analysed species, and no significant differences in the mean ellipticity occur (p>0.0167; Table S1). The bivariate plot of coccolith width versus coccolith ellipticity for the three studied species shows an increase with decreasing coccolith width (Fig. 3g).
3.4 Comparison with previous Biscutaceae measurements
The comparison of the observed size distributions measured in this paper to those published by Mattioli et al. (2004) shows a small overlap in coccolith length and width between Similiscutum giganteum sp. nov. and the slightly smaller Biscutum grande (Fig. 4a and b). The optical behaviour of these two species is, however, very different. Similiscutum giganteum displays a light grey, striated distal shield in optical-microscope crossed polars, without a prominent inner bright “collar”, similar to S. finchii and S. novum; on the other hand, the Biscutum grande uni-cyclic distal shield appears homogeneously dark grey and possesses a white, birefringent “collar” surrounding the central area (Bown, 1987a; Mattioli et al., 2004).
New biometric analyses performed on S. finchii and S. novum and on a large new species of Similiscutum, including coccolith length and width and their average values, allow an easy differentiation of the new species from the cluster composed of S. finchii and S. novum. From a morphological and biometrical point of view, S. finchii and S. novum show similar features, both being medium-sized, normal to broadly elliptical coccoliths (Mattioli et al., 2004). Similiscutum novum is, however, slightly smaller in size and less elliptical and has a smaller central area. In SEM (scanning electron microscope) pictures (like for the holotype; Fig. 5), the central area of S. finchii is lenticular, elongated and narrowly elliptical, while in S. novum the central area is rather sub-circular (see the holotype in Fig. 5). In LM (light microscope) crossed polars, the central area of S. finchii clearly appears elongated and sub-rectangular (Plate 2, figs. 1–4), while in S. novum it is rather squared (Plate 2, figs. 5–8). The different stratigraphical ranges reported for S. finchii and S. novum (Bown and Cooper, 1998; Mattioli and Erba, 1999; Ferreira et al., 2019) are a further argument to keep these two species separated.
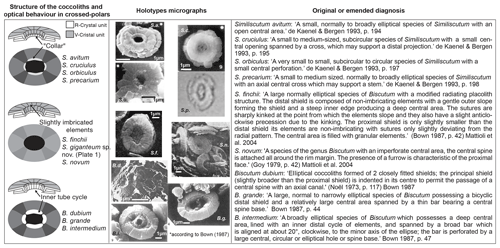
Figure 5In order to effectively compare Similiscutum giganteum sp. nov. to other Lower Jurassic Biscutaceae, the simplified structure in optical microscopes and SEMs (left column), the holotype micrographs (middle column), and the original or emended diagnoses (right column) are reported. An explanation of abbreviations is as follows: S.a. = Similiscutum avitum; S.c. = Similiscutum cruciulus; S.o. = Similiscutum orbiculus; S.p. = Similiscutum precarium; S.f. = Similiscutum finchii; S.n. = Similiscutum novum; B.d. = Biscutum dubium; B.g. = Biscutum grande; B.i. = Biscutum intermedium.
The central-area length and width and their means allow the differentiation of the large morphotype of Similiscutum from S. novum but not from S. finchii. The relative proportion of the central area with respect to the coccolith size (ratios “central-area length over coccolith length” and “central-area width over coccolith width”) and the coccolith ellipticity (ratio “coccolith length over coccolith width”) cannot be confidently used as diagnostic parameters for differentiating between these three morphotypes of Similiscutum.
Taking into account only size differences, the very large morphotype of Similiscutum had been previously considered to be a big morphotype of S. finchii (a probable ecophenotype), since they exhibit a similar optical behaviour. In fact, in the literature, a differentiation was made between small and large specimens of S. finchii by several authors (e.g. Bown and Cooper, 1998; Menini et al., 2019; Chaumeil Rodriguez et al., 2022). However, the original diagnosis of S. finchii (“A species of Biscutum with a large central area and no spine” (Crux, 1984, p. 168)) does not satisfactorily apply to the new species described here, which has a central area smaller than that of S. finchii and a cross structure inside it. On the basis of a combination of differences of size and of central-area shape and structure, Similiscutum giganteum sp. nov. is introduced here. This is also considerably different in size and geometry form other species of Biscutaceae (for a synthesis, see Fig. 5).
Taxonomic description
-
Similiscutum giganteum sp. nov.
Plate 1, figs. 1–9
-
1969 Palaeopontosphaera crucifera Prins, pl. 2, fig. 10 (nomen nudum).
-
1969 Palaeopontosphaera veterna Prins, pl. 2, fig. 9 (nomen nudum).
-
1987 Biscutum finchii Crux, 1984, emend. Bown, 1987a. Bown (partim), pl. 13, figs. 21–22.
-
1998 Biscutum finchii (Crux, 1984, emend. Bown, 1987a) de Kaenel and Bergen, 1993. Bown and Cooper, pl. 4.12, figs. 13–14 (large morphotype).
-
2002 Biscutum finchii (Crux, 1984, emend. Bown, 1987a) de Kaenel and Bergen, 1993. Perilli and Comas-Rengifo, pl. 1, fig. 12.
-
2006 Similiscutum giganteum Mailliot, p. 234, pl. 1, figs. 1–6, unpublished thesis.
-
2006 Biscutum finchii (Crux, 1984) Bown, 1987a. Perilli and Duarte, pl.1, figs. 7–8.
-
2008 Biscutum finchii (Crux, 1984) Bown, 1987a. Fraguas et al., pl. 1, fig. 10.
-
2014 Similiscutum giganteum Mailliot, 2006. Reolid et al., fig. 6.
-
2016 Similiscutum giganteum Mailliot, 2006. da Rocha et al., fig. 7.9–10.
-
2019 Similiscutum aff. S. finchii (Crux, 1984, emend. Bown, 1987a) de Kaenel and Bergen, 1993. Menini et al., pl. 2 (large, SN3.57B)
-
2019 Similiscutum finchii (Crux, 1984, emend. Bown, 1987a) de Kaenel and Bergen, 1993. Ferreira et al., pl. 2 (Peniche 61).
-
2022 Large Similiscutum aff. finchii (Crux, 1984, emend. Bown, 1987a) de Kaenel and Bergen, 1993. Chaumeil Rodríguez et al., pl. 2, fig. 15.
Derivation of name. From Latin giganteus, gigantic.
Diagnosis. A very large, broadly elliptical to elliptical species of Similiscutum with a bulky cross spanning the central area. The central area generally exhibits a lenticular shape and is quite reduced compared to the coccolith size.
Holotype. Plate 1, fig. 1 (fig. 1a – CPs: crossed polars, fig. 1b – PPs: parallel polars).
Type locality. Peniche (Ponta do Trovão section), Portugal.
Type level. Sample Pen08-Do2003 (= base of the “transition beds” or “couches de passage” = Bed 15a of Mouterde, 1955) in the uppermost Pliensbachian (Emaciatum ammonite Zone).
Repository: Collections de Géologie de Lyon with the FSL no. 766528.
Description. This coccolith is caracterised by a modified radiating placolith structure, similar to S. finchii and S. novum. The sutures between elements of distal shield often show important kinking. A robust cross spans the lozenge-like central area. The lenticular central-area shape is particularly visible in proximal view.
Dimensions. Minimum and maximum measured lengths are 6.32–9.93 µm (mean is 8.23 µm); width is 5.08–8.73 µm (mean is 6.91 µm); central-area length is 1.61–3.53 µm (mean is 2.35 µm); central-area width is 1.31–2.21 µm (mean is 1.78 µm).
Holotype (Plate 1, fig. 1) measurements are 8.10 µm (length) – 7.01 µm (width).
Range. Late Pliensbachian (Margaritatus ammonite Zone) to late Toarcian (Meneghinii ammonite Zone) (Ferreira et al., 2019).
Occurrence. Portugal (Peniche, this work); Spain (Camino in Perilli and Comas-Rengifo (2002); La Cerradura in Reolid et al. (2014)); France (Saint-Paul-des-Fonts and Tournadous in Mailliot (2006); ANDRA HTM-102 borehole in Lorraine from personal observation by Emanuela Mattioli (2018); Anse Saint Nicolas in Menini et al. (2019)); Argentina (Picun Leufu in Bown (1987a), Bown and Cooper (1998) and Chaumeil-Rodriguez et al. (2022)).
Discussion. The name Similiscutum giganteum was informally used in Mailliot (2006; PhD thesis) but was never formally published. The same name was informally used in Reolid et al. (2014) and da Rocha et al. (2016).
According to biometric data presented in this paper, Similiscutum giganteum sp. nov. is one of the largest Biscutaceae of the Lower Jurassic, along with Biscutum grande – however, the latter possesses a peculiarly different extinction pattern in optical microscope and a different structure as far as the inner “collar” is concerned. Bown (1987a) and then Bown and Cooper (1998) have differentiated between small and large morphotypes of Similiscutum finchii, with coccolith length ranging from 5.8 to 8.5 µm and coccolith width ranging from 4.8 to 7.0 µm. The large specimens described by these authors significantly differ from the original diagnosis of Similiscutum finchii because of their reduced central-area dimensions and more broad elliptical outline of the coccolith (pl. 4.12, figs. 13–14). De Kaenel and Bergen (1993) pointed out an important difference in size between specimens of Similiscutum finchii from Portugal, ranging from 5.5 to 8.0 µm, and specimens from Morocco, as small as 5.0 µm. Although not shown, it is not excluded that some of the larger specimens from Portugal noticed by De Kaenel and Bergen (1993) corresponded to Similiscutum giganteum sp. nov. Mattioli et al. (2004; p. 16) stated that the size range of Similiscutum finchii, as evidenced by biometrics, “…falls at the small end of the range of sizes reported in the literature…”, with a majority of specimens ranging between 4.0 and 5.1 µm. The specimens of Similiscutum finchii coming from Portugal and measured in the present work are indeed larger than in other papers, ranging from 4.28 to 6.17 µm, with a mean at 5.23 µm. The presence of over-calcified, robust coccoliths seems to be a common pattern in Peniche (da Rocha et al., 2016). However, the large S. finchii mentioned by Bown (1987a), Bown and Cooper (1998) and de Kaenel and Bergen (1993) rather corresponds to Similiscutum giganteum sp. nov.
Similiscutum giganteum sp. nov. is also easily distinguished from other Similiscutum because of its peculiar, lozenge-like central-area shape, visible in both optical-microscope and SEM images (Plate 1, figs. 4–6 and 8–9). Similiscutum novum generally exhibits a small, square to sub-rectangular central-area shape in optical microscopes (Plate 2, figs. 5–8). Similiscutum finchii usually displays a sub-rectangular to rectangular, elongated central-area shape (Plate 2, figs. 1–4). Similiscutum giganteum sp. nov. also possesses a robust cross in its central area visible in optical microscopes (Plate 1, figs. 1a, 3a, 4b, 5a) and SEMs (Plate 1, figs. 7 and 8). Although this structure can be (partially) broken (e.g. Plate 1, fig. 8), its insertion points on the inner part of the central area are generally well visible. The occurrence of a cross in the central area is a diagnostic parameter for the characterization of this species. The structure of the central area is different for the other Similiscutum species. In the description of Similiscutum novum (Bown, 1987a; p. 41), it is mentioned that “…the central area of the proximal shield is filled with granular calcite forming a funnel-like structure which protrudes as the spine/tube on the distal side. Only rarely is the central, hollow tube found as a fully developed spine, and this may represent a dimorphic feature or simply be due to preservation…”. The original diagnosis of Similiscutum finchii (Crux, 1984; p. 168) reports “…a species of Biscutum with a large central area and no spine; the central area is filled with irregular granular calcite…”. The emended diagnosis of Bown (1987a; p. 42) states that “…the central area is filled with granular elements…”. De Kaenel and Bergen (1993; p. 877) report for S. finchii that “…in cross-polarized light, a bright transverse bar is often observed when specimens are oriented 45∘ to the polarizing direction; this optical feature results from a thickening of the central plate elements…”. Finally, Mattioli et al. (2004; p. 25) noticed that the central area of S. finchii “…can be crossed by simple structures…”. However, the occurrence of a cross in the central area of S. finchii is never mentioned in the literature.
Differentiation. Similiscutum giganteum can be distinguished from S. finchii and S. novum by its larger size and by the occurrence of a well-developed cross in the lenticular central area. Similiscutum giganteum can be distinguished from the large species Biscutum grande by its uni-cyclic distal shield, by its somehow larger size, by the existence of kinking sutures on the distal shield elements and, especially, by the absence of a typical very bright inner tube cycle and of the bridge structure which spans the central area of B. grande. The latter may sometimes be characterized by a well-developed cross in its central area, as shown by Menini et al. (2019) (plate 2, LAL-18).
Since the 90s, the Jurassic Biscutaceae have been the object of taxonomic revisions, with the introduction of a new genus and several species, which allowed a better biochronological subdivision of the Jurassic period. Since then, the first occurrence of Similiscutum has been used to define the lowermost nannofossil zone of the Pliensbachian stage, and this event is now used in the Geologic Time Scale 2020. This paper reports on biometric analyses performed on three species of Similiscutum, namely S. novum, S. finchii and a new, very large morphotype of Similiscutum recorded in upper Pliensbachian and Toarcian strata. Because of morphological plasticity shown by S. finchii, which presents small and large morphotypes, this new and very large Similiscutum was erroneously attributed to S. finchii. In fact, as shown by our new biometric data, the original diagnosis of S. finchii does not apply to this taxon that has to be considered as a new species. On the basis of combined differences of sizes and of central-area shape and structure, a new taxon, Similiscutum giganteum sp. nov., is thus introduced here for designating very large coccoliths, which represent amongst the largest known Jurassic Biscutaceae.
All the slides containing nannofossils studied and illustrated in this paper are stored in the Collections de Géologie de Lyon Repository (France) with the label FSL and a number. All the data used in this paper are shown in the Table S1 and S2 in the Supplement.
The supplement related to this article is available online at: https://doi.org/10.5194/jm-42-1-2023-supplement.
SM, EM and BP performed the sampling. SM performed the sample preparation, light–microscope imaging and biometrics. The species were determined by SM under the supervision of EM. MCR contributed with the taxonomic revision. SM prepared the article and figures with contributions from the co-authors. All authors contributed to writing and editing the article.
At least one of the (co-)authors is a member of the editorial board of Journal of Micropalaeontology. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors warmly thank Fabienne Giraud and Serge Elmi for the constructive discussions and review of an earlier version of this paper.
Samuel Mailliot was funded by MRT of the French Ministry of Research. Financial support was provided by the Projet ATIP (CNRS PG/MR/No.2002.053).
This paper was edited by Kirsty Edgar and reviewed by Angela Fraguas and Jeremy Young.
Akaike, H.: Information theory and an extension of the Maximum Likelihood principle, edited by: Petrov, B. N. and Csaki, F., 2nd International Symposium on Information Theory, Akademia Kiado, Budapest, 267–281, 1973.
Black, M.: Coccoliths of the Speeton Clay and Sutterby Marl, Proceedings of Yorshire Geological Society, 38, 381–424, 1971.
Bown, P. R.: Taxonomy, evolution and biostratigraphy of Late Triassic-Early Jurassic calcareous nannofossils, Spec. Pap. Palaeontol., 38, 1–118, 1987a.
Bown, P. R.: The structural development of Early Mesozoic coccoliths and its evolutionary and taxonomic significance, Abh. Geol. B.-A., 39, 33–49, 1987b.
Bown, P. R. and Cooper, M. K. E.: Jurassic, edited by: Bown, P. R., Calcareous Nannofossil Biostratigraphy, British Micropalaeontological Society Publication Series, Kluver Academic Publishers, 34–85, https://doi.org/10.1007/978-94-011-4902-0, 1998.
Chaumeil Rodríguez, M., Mattioli, E., and Pérez Panera, J. P.: Lower Jurassic calcareous nannofossil taxonomy revisited according to the Neuquén Basin (Argentina) record, J. Micropalaeontol., 41, 75–105, https://doi.org/10.5194/jm-41-75-2022, 2022.
Crux, J. A.: Biostratigraphy of Early Jurassic Calcareous Nannofossils from Southwest Germany, Neues Jahrb. Geol. P.-A., 169, 160–186, 1984.
da Rocha, R., Mattioli, E., Duarte, L. V., Pittet, B., Elmi, S., Mouterde, R., Cabral, M. C., Comas-Rengifo, M. J., Gómez, J. J., Goy, A., Hesselbo, S. P., Jenkyns, H. C., Littler, K., Mailliot, S., Veiga de Oliveira, L. C., Osete, M. L., Perilli, N., Pinto, S., Ruget, C., and Suan, G.: Base of the Toarcian Stage of the Lower Jurassic defined by the Global Boundary Stratotype Section and Point (GSSP) at the Peniche section (Portugal), Episodes, 39, 460–481, 2016.
de Kaenel, E. and Bergen, J. A.: New Early and Middle Jurassic coccolith taxa and biostratigraphy from the eastern proto-Atlantic (Morocco, Portugal and DSDP Site 547 B), Eclogae Geol. Helv., 86, 861–907, 1993.
Favre, E., Escarguel, G., Suc, J.-P., Vidal, G., and Thévenod, L.: A contribution to deciphering the meaning of AP/NAP with respect to vegetation cover, Rev. Palaeobot. Palyno., 148, 13–35, 2008.
Ferreira, J., Mattioli, E., Sucherás-Marx, B., Giraud, F., Duarte, L. V., Pittet, B., Suan, G., Hassler, A., and Spangenberg, J. E.: Western Tethys Early and Middle Jurassic calcareous nannofossil biostratigraphy, Earth Sci. Rev., 197, 102908, https://doi.org/10.1016/j.earscirev.2019.102908, 2019.
Fraguas, A., Comas-Rengifo, M. J., and Perilli, N.: Pliensbachian calcareous nannofossils of the Santotis section (Basque-Cantabrian Basin, N Spain), Atti della Società Toscana di Scienze Naturali, Serie A, 113, 49–56, 2008.
Fraguas, A., Comas-Rengifo, M. J., and Perilli, N.: Calcareous nannofossil biostratigraphy of the Lower Jurassic in the Cantabrian Range (Northern Spain), Newsl. Stratigr., 48/2, 179–199, https://doi.org/10.1127/nos/2015/0059, 2015.
Fraguas, A., Comas-Rengifo, M. J., Goy, A., and Gómez, J. J.: Upper Sinemurian–Pliensbachian calcareous nannofossil biostratigraphy of the E Rodiles section (Asturias, N Spain): a reference section for the connection between the Boreal and Tethyan Realms, Newsl. Stratigr., 51/2, 227–244, https://doi.org/10.1127/nos/2017/0401, 2018.
Hammer, Ø., Harper, D. A. T., and Ryan, P. D.: PAST: paleontological statistics software package for education and data analysis, Palaeontol. Electron., 4, 1–9, 2001.
Hesselbo, S. P., Ogg, J. G., and Ruhl, M. (with contributions by Hinnov, L. A. and Huang, C. J.): The Jurassic Period, in: Geologic Time Scale 2020, Vol. 2, edited by: Gradstein, F. M., Ogg, J. G., Schmitz, M., and Ogg, G., 955–1021, https://doi.org/10.1016/B978-0-12-824360-2.00026-7, 2020.
Mailliot, S.: Production carbonatée pélagique par les nannofossiles calcaires au cours de l'événement anoxique du Toarcien inferieur, PhD thesis, Université Claude Bernard Lyon1, 221 pp. + 2 Annexes, 2006.
Mattioli, E. and Erba, E.: Biostratigraphic syntesis of calcareous nannofossil events in the Tethyan Jurassic, Riv. Ital. Paleontol. S., 105, 343–376, 1999.
Mattioli, E., Pittet, B., Young, J. R., and Bown, P. R.: Biometric analysis of Pliensbachian-Toarcian (Lower Jurassic) coccoliths of the family Biscutaceae: intra- and inter-specific variability versus palaeoenvironmental influence, Mar. Micropaleontol., 52, 5–27, 2004.
Menini, A., Mattioli, E., Spangenberg, J. E., Pittet, B., and Suan, G.: New calcareous nannofossil and carbon isotope data for the Pliensbachian/Toarcian boundary (Early Jurassic) in the western Tethys and their paleoenvironmental implications, Newsl. Stratigr., 52, 173–196, https://doi.org/10.1127/nos/2018/0476, 2019.
Mouterde, R.: Le Lias de Peniche, Direcção geral de minas e seviços geológicos, Lisboa, t. XXXVI das Comunicaçaos dos Serviços Geologicos de Portugal, 1–33 + 4 pl., CDU 551.762.1 (469.323.25), 1955.
Perilli, N. and Comas-Rengifo, M. J.: Calibration of Pliensbachian calcareous nannofossil events in two ammonite-controlled sections from Northern Spain (Basque–Cantabrian area), Riv. Ital. Paleontol. S., 108, 133–152, https://doi.org/10.13130/2039-4942/5463, 2002.
Perilli, N. and Duarte, L. V.: Toarcian nannobiohorizons from Lusitanian Basin (Portugal) and their calibration against ammonite zones, Riv. Ital. Paleontol. S., 112, 417–434, https://doi.org/10.13130/2039-4942/6350, 2006.
Peti, L., Thibault, N., Clémence, M.-E., Korte, C., Dommergues, J.-L., Bougeault, C., Pellenard, P., Jelby, M. E., and Ullmann, C. V.: Sinemurian–Pliensbachian calcareous nannofossil biostratigraphy and organic carbon isotope stratigraphy in the Paris Basin: Calibration to the ammonite biozonation of NW Europe, Palaeogeogr. Palaeocl., 468, 142–161, https://doi.org/10.1016/j.palaeo.2016.12.004, 2017.
Reolid, M., Mattioli, E., Nieto, L. M., and Rodríguez-Tovar, F. J.: The Early Toarcian Oceanic Anoxic Event in the External Subbetic (Southiberian Palaeomargin, Westernmost Tethys): Geochemistry, nannofossils and ichnology, Palaeogeogr. Palaeocl., 411, 79–94, 2014.
Suchéras-Marx, B., Mattioli, E., Pittet, B., Escarguel, G., and Suan, G., Astronomically-paced coccolith size variations during the early Pliensbachian (Early Jurassic), Palaeogeogr. Palaeocl., 295, 281–292, 2010.
Wiggan, N. J., Riding, J. B., Fensome, R. A., and Mattioli, E.: The Bajocian of the Mid-Jurassic: a critical step in the early Mesozoic phytoplankton radiation, Earth Sci. Rev., 180, 126–146, https://doi.org/10.1016/j.earscirev.2018.03.009, 2018.
Young, J. R., Kucera, M., and Chung, H.-W.: Automated biometrics on captured light microscope images of coccoliths of Emiliania huxleyi, edited by: Moguilevsky, A. and Whatley, R., Microfossils and Oceanic Environments, University of Aberystwyth Press, Aberystwyth, 261–280, ISBN 0903878747, 1996.
Young, J. R., Bergen, J. A., Bown, P. R., Burnett, J. A., Fiorentino, A., Jordan, R. W., Kleijne, A., van Niel, B. A., Romein, A. J. T., and von Salis, K.: Guidelines for coccolith and calcareous nannofossil terminology, Palaeontology, 40, 875–912, 1997.