the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Leisure boat harbours, hidden alien species, and pollution: a case study of Hinsholmskilen harbour (Gothenburg, Sweden)
Irina Polovodova Asteman
Emilie Jaffré
Agata Olejnik
Maria Holzmann
Mary McGann
Kjell Nordberg
Jean-Charles Pavard
Delia Rösel
Magali Schweizer
Small leisure boat harbours have important aesthetic and recreational values in any country with a coastline. In Sweden, there are about 860 000 leisure boats, which is one of the world's highest numbers in relation to the country's population. However, small boat harbours also present a wide range of environmental problems, including the introduction of alien species and high pollution. In this study, we investigated the ecological quality status (EcoQS) of the Hinsholmskilen small boat harbour, located southwest of the city of Gothenburg (Sweden). We performed a reconnaissance survey of the harbour's previously unstudied benthic foraminiferal communities and analysed surface sediment (0–2 cm) samples for potentially toxic elements: copper (Cu), zinc (Zn), lead (Pb), cobalt (Co), nickel (Ni), chromium (Cr), mercury (Hg), and arsenic (As). The results show that, based on the total benthic foraminiferal distribution (dead and live specimens), the assemblages in Hinsholmskilen harbour represent a typical European estuarine community with highly abundant Ammonia and Elphidium species. Based on molecular and morphological data, we report the presence of two alien and putatively invasive species likely originating from Asia: Trochammina hadai and Ammonia confertitesta (phylotype T6). Both species have recently been identified elsewhere on the Swedish west coast based on molecular and morphological data but do not have a well-known distribution. The sediment analysis for potentially toxic elements showed that the harbour has good to high EcoQS corresponding to no or little deviation from reference conditions for Cd, Co, Ni, and Pb distribution. Some of the contaminants (Pb, As, Zn, and Cr) showed poor to bad EcoQS in the innermost harbour in proximity to high-pressure cleaning plants, where boats are usually lifted, cleaned, and prepared for winter storage on land. Finally, Cu and Hg showed consistently bad and poor EcoQS all over the harbour, reflecting the use of both metals as biocides in antifouling boat paints.
- Article
(14128 KB) - Full-text XML
-
Supplement
(8104 KB) - BibTeX
- EndNote
Marine alien and invasive species are a growing concern because they can threaten ecosystem goods and services through biodiversity loss, habitat modifications, changes in community structure, and substantial economic losses (Bax et al., 2003; Katsanevakis et al., 2014). Alien species are often transported with ship ballast water, anchor mud, hull fouling, or commercial aquaculture (McGann et al., 2019). The North Sea and the Skagerrak and Kattegat regions are intensive shipping areas and already host a high number of marine alien and invasive species (Staehr et al., 2020). However, there is a substantial “size bias” in marine alien and invasive species research, with a major focus on larger organisms. Little is known about introduced eukaryotic microorganisms (Pettay et al., 2015; McGann et al., 2019; Roy et al., 2023), or so-called “hidden aliens”, even though microbes spread more easily (Hülsmann and Galil, 2002), are prone to dormancy (Kosakyan et al., 2012), and can establish large population sizes (Epstein and López-García, 2008), which all likely explain their higher adaptive capacity. Benthic foraminifera are unicellular eukaryotes, which play an important role in sediment bio-irrigation and biogeochemical cycles (e.g. Piña-Ochoa et al., 2010; Salonen et al., 2019; Glock et al., 2025). Some foraminifera have different cellular respiration processes as compared to the majority of meiofaunal organisms (Maciute et al., 2023), likely due to presence of other metabolic strategies such as denitrification (Glock, 2023), anaerobic dephosphorylation and fermentation (Orsi et al., 2020; Gomaa et al., 2021), and aerobic fixation of carbon and nitrogen through retention of algal chloroplasts (LeKieffre et al., 2018). These strategies make some alien and invasive foraminiferal species remarkable extremophiles, able to survive adverse environmental conditions, such as severe hypoxia and anoxia (Choquel et al., 2021; Glock et al., 2025), when native species (lacking those strategies) would migrate or perish. Today, foraminiferal alien and invasive species are known from the western and eastern coasts of North America (e.g. McGann et al., 2019; McGann and Holzmann, 2024; Goetz et al., 2025), the Mediterranean Sea (e.g. Hyams-Kaphzan et al., 2008; Langer et al., 2012), the Baltic Sea (Schweizer et al., 2011; Groeneveld et al., 2018), the North Sea (Polovodova Asteman and Schönfeld, 2016; Charrieau et al., 2018; Deldicq et al., 2019; Choquel et al., 2021; Francescangeli et al., 2021), the coasts of the French Atlantic Ocean (e.g. Bouchet et al., 2007, 2023; Pavard et al., 2023a, b; Fouet et al., 2024), New Zealand (e.g. Hayward, 1997; Grenfell et al., 2007), Australia (Tremblin et al., 2021), Brazil (e.g. Eichler et al., 2018), and Argentina (Calvo-Marcilese and Langer, 2010). Studies performed over the last 3 decades report growing abundances of alien and invasive species (including foraminifera) in small leisure boat harbours, suggesting that these harbours may be a hotspot for secondary species introductions, facilitating their spread over larger areas (e.g. Arenas et al., 2006; Ferrario et al., 2017; Bouchet et al., 2023; Pavard et al., 2023a, b).
In contrast to a growing number of studies on coral reefs, mangrove forests, seagrass meadows, salt marshes and their associated alien species introductions, and other types of anthropogenic impacts, much less research to date has been performed on small leisure boat harbours. Sweden has a long and complex coastline with numerous archipelagos, and it is estimated that there are about 860 000 leisure boats in Sweden (Transportstyrelsen, 2021), which is one of the highest numbers in the world in relation to the country's population (Nordberg et al., 2022). Swedish coasts have many bays and fjords and a microtidal regime, keeping them protected from wind and waves, thus offering an opportunity to store leisure boats in small marinas and private piers. This, together with an ever-growing interest and demand to live close to the sea, has historically increased the number of leisure boat harbours. Being used for mainly aesthetic values and recreational activities, small boat harbours have been shown to be associated with the introduction of alien and invasive species, mainly through attachment to the boat anchors, engines, or hulls (Ferrario et al., 2017). Small boat harbours also present a wide range of pollution problems related to e.g. boat traffic, maintenance, and storage and to the use of antifouling paints preventing the growth of macroalgae, barnacles, and other aquatic organisms. As a result, the shallow harbour environment is often exposed to a cocktail of toxic pollutants in the form of antifouling paints, impregnating agents from the wooden docks, petrochemical products from boat engine exhausts, and potentially toxic elements, which all accumulate in the harbour sediments, posing a threat to shallow benthic ecosystems (e.g. Nordberg et al., 2012, 2022, 2025; Eklund et al., 2016; Egardt et al., 2017; Moksnes et al., 2019). Among other environmental problems associated with leisure boat harbours are shading from boats and boat docks; water-darkening due to sediment resuspension; increased hydrodynamic activity and underwater noise; microplastics from boat paints, mooring lines, and piers; and the release of combustion exhaust gases (Moksnes et al., 2019). For instance, sediment resuspension by boat propellers and harbour dredging has shown to decrease access to light for vegetation, e.g. eelgrass meadows providing important spawning and feeding grounds for many fish species (Dennison et al., 1993). The dredging and dumping of dredged sediment in shallower waters also removes and modifies the habitat for benthic organisms, with negative effects for biodiversity (e.g. Harvey et al., 1998; Desprez, 2000; Bolam, 2012; Rehitha et al., 2017). High waves and currents generated by boat traffic can cause bottom and beach erosion in shallow-water areas (Klein, 2007). Microplastics from worn ropes, fishing gear, boat paints, and plastic used in buoys and pier construction can adsorb organic pollutants and metals (Hirai et al., 2011; Brennecke et al., 2016) and can be taken up by marine organisms and transported in food webs (Farrell and Nelson, 2013). Combustion exhaust gases (incl. NOx, SOx, CO, CO2, and volatile organic compounds) can cause a wide array of health problems in both humans and aquatic organisms (Moksnes et al., 2019), spread polycyclic aromatic compounds (PAHs) in the water, and eventually accumulate in the sediment (Nordberg et al., 2022, 2024, 2025).
This study aims to assess the ecological status of the small boat harbour of Hinsholmskilen (Fig. 1), located southwest of the city of Gothenburg (Sweden). The goal of the study is 2-fold: (a) to perform a reconnaissance survey of the harbour's previously unstudied benthic foraminiferal communities with focus on two non-indigenous species, which both do not have a well-known distribution on the Swedish west coast, and (b) to map the distribution of trace metals and arsenic in the harbour surface sediments.
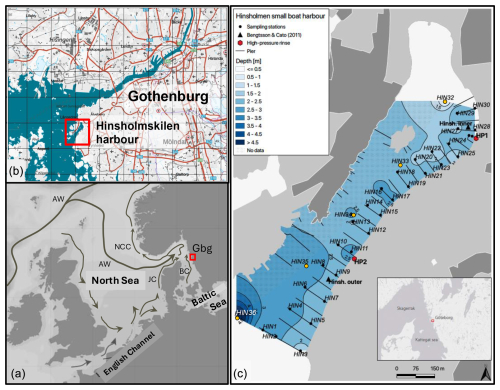
Figure 1Map of the study area. (a) Location of Hinsholmskilen harbour in the North Sea–Baltic Sea region. The prevailing currents are indicated as follows: JC – Jutland Current; BC – Baltic Current; NCC – Norwegian Coastal Current; and AW – Atlantic Water inflow. The abbreviation Gbg stands for the city of Gothenburg. (b) Location of the study area in the city of Gothenburg (map adapted and modified from SGU, 2023) facing the Kattegat and the Gothenburg archipelago in the west. (c) Sampling locations in Hinsholmskilen harbour, for which benthic foraminifera, metal, and arsenic data were obtained in this study: manual sampling from the piers (black circles) and sampling by motorboat (orange circles). HP1 and HP2 stand for high-pressure cleaning stations, where boats are lifted and rinsed with pressurized water.
The Hinsholmskilen leisure boat harbour (Fig. 1) is located to the southwest of the city of Gothenburg, on the west coast of Sweden. The harbour is in a shallow bay, where water circulation is mostly driven by coastal currents and prevailing winds. The sea level changes are controlled by tidal activity, winds, and changes in atmospheric pressure. Low pressure and headwinds cause elevated sea level in the area, whilst high pressure and offshore winds decrease sea level in the bay. Prevailing winds in the study area are westerlies and southwesterlies. Hinsholmskilen harbour faces the adjacent Kattegat and is therefore influenced by the major surface currents in the area: the outflowing Baltic Current (S= 20–30) and the inflowing Jutland Current (S= 31–34) (Danielssen et al., 1997; Fig. 1a herein). Hinsholmskilen harbour is surrounded by areas with exposed bedrock, which consist of granodiorite and tonalite. The main sediment type in the harbour is represented by thick glacial clay covered with a fine layer of clay, gyttja clay, and clayey gyttja. Gyttja clay is a Scandinavian term for an organic-rich clay (TOC < 50 %) which is fine-textured, very soft, and hydrous; has a dark-greenish to black colour; and is typically found in sheltered bays and fjords (Wetzel, 2001). The recent sediments in Hinsholmskilen harbour consist mainly of gyttja clay and fine to medium sand (SGU, 2002: Series Ba, 59:4). Below a thin oxic sediment layer, the sediment is anoxic and rich in molluscan shell debris. Sediment accumulation rates in Hinsholmskilen harbour are estimated to be 0.5 cm yr−1 (Bengtsson and Cato, 2011).
Hinsholmskilen harbour is the second-largest marina for leisure boats in the Gothenburg region. It was built in 1966 and has the capacity to host 1500 boats during the summer high season whilst providing storage for 850 boats on land during the winter (Hinsholmens Båtklubb, 2023 http://www.hinsholmen.se, last access: 5 June 2023). There are 28 piers in the harbour, mostly made of wood, with a few built of concrete, and with a few floating docks. There are also two cleaning plants equipped with a high-pressure water system with drainage and filtering facilities connected to septic chamber tanks to collect rinse water and residue of paint rinsed off from boats being lifted onto land prior to winter storage. Due to its extensive use, Hinsholmskilen harbour has environmental issues related to pollution by metals, organotin compounds, Irgarol, and PAHs (Bengtsson and Cato, 2011; Bengtsson and Wernersson, 2012; Eklund et al., 2016). A survey performed in 2010 at two stations in the harbour, Hinsholmskilen Inner and Hinsholmskilen Outer (Fig. 1), showed that, even though most metals decreased in the area between the 1990s and 2010, levels of the antifouling agent tributyltin (TBT) doubled from the 1990s to 2010 in the inner harbour (Bengtsson and Cato, 2011). High pollution in the inner-harbour sediments likely results from land runoff through sewage and stormwater outlets draining contaminated water from the high-pressure cleaning stations into the harbour. Analyses of sludge in the sewage wells, stormwater, and drainage wells near high-pressure cleaning stations showed that levels of zinc (Zn), copper (Cu), and the antifouling agents TBT and Irgarol exceed bad ecological quality status (EcoQS) class and that high contaminant concentrations in the sludge are similar to levels found in hazardous waste (Bengtsson and Wernersson, 2012).
3.1 Sediment sampling
This study is based on two sets of samples: one collected in November 2019 for the morphological and geochemical study and the other collected in May 2023 and May 2024 for the DNA study by using a Kajak–Brinkhurst corer (weight: 9 kg; core tube: 5 cm Ø). Sediment samples collected in November 2019 were mostly taken manually from the harbour piers, whilst five sediment samples were collected by using a small motorboat (Fig. 1). In total, 36 stations were visited to collect surface (0–2 cm) sediment samples for benthic foraminifera and potentially toxic elements (Supplements S1–S3 and S5). Upon arrival to the laboratory, all samples were frozen and freeze-dried. Many samples contained a lot of molluscan shell debris and had to be dry-sieved over a 1000 µm plastic sieve to obtain homogeneous sediment for metal analyses. For the 2023 and 2024 samples, surface sediments (0–3 cm) were collected manually from the piers at the stations HIN1, HIN2, HIN12, and HIN27 (Fig. 1).
From the November 2019 samples, 23 samples were analysed for total (living + dead) benthic foraminifera, whilst 36 samples were analysed for potentially toxic elements, such as arsenic (As), cadmium (Cd), cobalt (Co), nickel (Ni), lead (Pb), zinc (Zn), mercury (Hg), chromium (Cr), and copper (Cu). The major goal during the sampling campaign in 2019 was to map the distribution of these potentially toxic elements in the harbour; therefore, no staining with rose bengal for living foraminifera was done. Also, no hydrographic measurements (temperature, salinity, and oxygen) were taken at the time of sampling.
3.2 Foraminifera: morphological analysis
To make a reconnaissance of recent foraminiferal assemblages in this previously unstudied harbour, the unstained sediment samples taken in 2019 were dry-weighed and washed over 63 and 1000 µm sieves. The sediment residue > 63 µm was air-dried at 50 °C and picked under a stereomicroscope for benthic foraminifera, which were identified to the lowest possible taxonomic level. Selected species were mounted on aluminium stubs, coated with gold, and imaged with a Hitachi S-3400N scanning electron microscope (SEM) at the Department of Earth Sciences, University of Gothenburg. Since this study reports foraminiferal data as total assemblages, these data could not be used for the harbour EcoQS assessment, which should be based on living (stained) assemblages (Murray, 2000). However, total assemblages can provide a first glimpse into previously unstudied foraminiferal fauna in Hinsholmskilen harbour. During foraminiferal morphological examination, unknown Trochammina and Ammonia species (Plates 1–2) were discovered in the samples. Those species were hypothesized to represent the putatively invasive species Trochammina hadai Uchio (1962) (McGann et al., 2000) and Ammonia confertitesta Zheng, 1978 (phylotype T6: Hayward et al., 2021), both originating from Asia (McGann et al., 2000; Hayward et al., 2004; Schweizer et al., 2011; McGann and Holzmann, 2024).
The first attempt to identify Trochammina individuals was made on their agglutinated shells to test if they were organic-cemented or carbonate-cemented, following Tremblin et al. (2021). This was done by testing for a shell reaction with 2 % HCl, which failed and suggested the presence of organic cement in shell matrix. The usual method to discriminate Ammonia species uses the two main morphological criteria: the elevation of sutures and the mean pore size diameter (Richirt et al., 2019, 2021). However, the Ammonia tests collected in Hinsholmskilen harbour in 2019 were showing signs of dissolution and could not be identified morphologically.
To identify these taxa more precisely, new samples were collected in late May 2023 and 2024 from the Trochammina- and Ammonia-rich stations HIN1, HIN2, HIN12, and HIN27 (Fig. 1) to obtain living representatives for scanning electron microscopy, light imaging, and single-cell DNA extraction. In addition to sediment, ambient seawater was collected from the field. Living foraminifera were picked within 1–2 d after sampling. Small aliquots of sediment were washed over a 63 µm sieve with pressurized seawater, and the > 63 µm residue was collected in a Petri dish with some seawater and examined for foraminifera under a stereomicroscope. Living Trochammina and Ammonia individuals with bright-yellow to orange cytoplasm were transferred into a new Petri dish and left for 30 min at room temperature, and their vitality was assessed based on pseudopodial activity through a so-called “crawling test”. Active living individuals were rinsed in distilled water, air-dried, and sent for DNA extractions (Trochammina specimens to the University of Geneva and Ammonia specimens to the University of Angers) to verify the presence of both non-indigenous species by molecular methods.
3.3 Foraminifera: DNA extraction, PCR amplification, and sequencing
Eight Trochammina hadai specimens (isolates 22013–22020, Table 1) were photographed using a Leica M205 C microscope fitted with a Leica DFC450 C camera at the University of Geneva prior to DNA extraction (Plate 1). DNA was extracted individually using guanidine lysis buffer (Pawlowski, 2000). Semi-nested PCR amplification was carried out for the 18S barcoding fragment of foraminifera (Pawlowski and Holzmann, 2014) using primers s14F3 (5“acgcamgtgtgaaacttg3”)-sB (5“tgatccttctgcaggttcacctac3”) for the first amplification and primers s14F1 (5“aagggcaccacaagaacgc3”)-sB for the second amplification. A total of 35 and 25 cycles were performed for the first and the second PCR, with an annealing temperature of 50 and 52 °C, respectively. For seven specimens (22013–22019), positive amplification results were obtained. The amplified PCR products were purified using the High Pure PCR Cleanup Micro Kit (Roche Diagnostics). Sequencing reactions were performed using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) and analysed on a 3130XL genetic analyser (Applied Biosystems). The resulting sequences were deposited in the NCBI/GenBank database. Isolate and accession numbers are specified in Table 1.
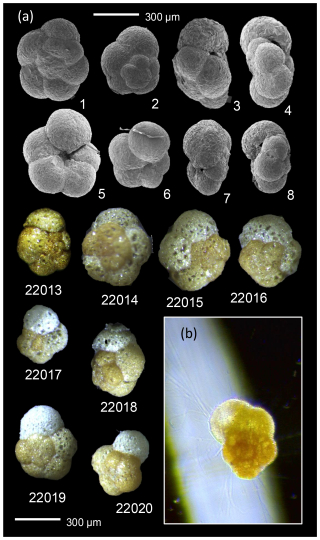
Plate 1Scanning electron microscope (SEM) and light microscopic images of Trochammina hadai. (1–8) SEM images of subfossil T. hadai recovered from non-stained foraminiferal samples taken in 2019. 22013–22020: Living individuals of T. hadai sampled in 2023 and extracted for DNA (see Fig. 2 herein). Image in frame: individual 22019 with active pseudopodia.
A total of 29 Ammonia sp. individuals were collected for molecular identification (isolates HH001–HH029, Table 1). Their test morphology was documented with a scanning electron microscope (SEM; Hitachi TM4000), and two images were taken: full specimens in spiral view and a 1000× magnification of the penultimate chamber of the spiral side (Supplement S4). Morphometrical parameters were measured on the 1000× image of the penultimate chamber following the semi-automated method described by Petersen et al. (2016) to identify Ammonia morphologically (Richirt et al., 2019). Thereafter, each individual Ammonia was crushed and extracted in DOC buffer (Pawlowski, 2000). A fragment of approximatively 500 bp within the barcode for foraminifera (Pawlowski and Holzmann, 2014) was amplified in two steps with a nested PCR. The first step consisted of the amplification of a fragment of 1000 bp using the primers s14F3 and J2 (Pawlowski, 2000; Darling et al., 2016). The second step of the nested PCR was performed using the primers s14F1 and N6 (Pawlowski, 2000). A total of 40 and 25 cycles were performed for the first and the second PCR, with an annealing temperature of 50 and 52 °C, respectively. Altogether, 26 of the samples amplified positively, and 16 of them were sent to Macrogen Europe (Amsterdam) for Sanger sequencing. Among these 16 samples, 10 gave good-quality sequences, which were deposited in the NCBI/GenBank database. Isolate and accession numbers are specified in Table 1. A further 6 samples gave low-quality sequences with background noise and thus were not published.
3.4 Foraminifera: phylogenetic analysis
The obtained sequences of T. hadai were added to 26 sequences belonging to 22 textulariids and 4 Reophacidae that are part of the publicly available 18S database of rotaliid foraminifera (NCBI/Nucleotide; https://www.ncbi.nlm.nih.gov/nucleotide/, last access: 14 November 2024). All sequences were aligned using the default parameters of the Muscle automatic alignment option, as implemented in SeaView v. 4.3.3 (Gouy et al., 2010). The alignment contains 33 sequences with 1191 sites used for analyses.
The phylogenetic tree (Fig. 2) was constructed using maximum-likelihood phylogeny (PhyML 3.0) as implemented in ATGC: PhyML (Guindon et al., 2010). An automatic model selection by SMS (Lefort et al., 2017) based on the Akaike information criterion (AIC) was used, resulting in a GTR + R substitution model being selected for the analysis. The initial tree is based on BioNJ. Bootstrap values (BVs) are based on 100 replicates.
3.5 Sediment analysis for potentially toxic elements
For metal and arsenic analyses, all sediment samples were weighed and sent to the ALS Scandinavia AB laboratory in Luleå (Sweden). Prior to analyses, the samples were dissolved with HNO3 and were then analysed in an inductively coupled plasma sector field mass spectrometer (ICP-SFMS) according to the SS EN ISO 17294-1, 2 (mod) and EPA 200.8 (mod) standards. Metal and arsenic concentrations in the sediments were classified into five ecological quality status (EcoQS) classes (high, good, intermediate, poor, and bad) based on Swedish Environmental Protection Agency guidelines (Supplements S1–S2), which are defined based on the deviation from reference conditions (Naturvårdsverket, 2000). These guidelines use background levels (Supplements S2–S3) measured in Swedish coastal sediments reflecting pre-industrial levels generally found deeper than 55 cm depth in the sediment cores (Naturvårdsverket, 2000). The metal and arsenic distributions were plotted on maps by using the software QGIS 3.10. Spatial interpolation was used to interpolate a projection between the measured contaminant concentration data points. For this we used the inverse distance weighted (IDW) interpolation, which assumes that the influence of one point relative to another declines with a distance. Weighting of data points is controlled by a weighting power. The greater the power, the more influence the known values have. For this study, a power of 2 was used in the metal data interpolation.
3.6 Statistical analysis
Relative abundances of foraminiferal species (Supplement S5) were subjected to an initial detrended correspondence analysis in R and indicated a short gradient (1.6 SD). Therefore, a redundancy analysis (RDA) was applied on this dataset to see which environmental parameter best explained the distribution of foraminiferal species. Environmental parameter datasets (metals, As, and water depth) were mean-centred, and the foraminiferal species dataset was log(x+ 1)-transformed prior to the analysis. Moreover, a variance inflation factor (VIF) analysis was performed to remove the effect of multicollinearity of some environmental parameters on the explained variability in foraminiferal species. Consequently, Cr, Ni, and Pb data were removed from the RDA (VIF > 20). Permutation tests (n= 999) were then performed to determine the significance of the whole analysis by axis and terms (i.e. environmental parameters), and the adjusted explained variability R2 was determined.
4.1 Sediment description
Most sediment samples represented grey to greenish-brown gyttja clay rich in molluscan shell debris, whilst fine to medium sand was found outside the two cleaning plants HP1 and HP2 (samples HIN6, HIN11, HIN12, HIN23). Darker sediment was found outside HP1 (samples HIN22, HIN25–29). Retrieved sediment cores had a few-millimetre-thick layer of oxygenated sediment on top and a thick, heavily anoxic layer below, with a distinct hydrogen sulfide scent detected at some stations (HIN3, HIN16, HIN17, HIN20, HIN25). Estimated content of carbonate shell debris reached > 50 % at stations HIN7 and HIN21. High shell debris content was also found in the outermost part of the harbour (samples HIN1–3, 4, 6, 8).
4.2 Harbour foraminifera: species reconnaissance based on morphological analysis of total assemblages
From the 23 studied samples herein, 18 were rich in benthic foraminifera (149.7–591 ind. g−1, with 308.2 ind. g−1 on average). Species richness was low, with 10 species per sample on average (range 6–11). There were 5 samples (HN27, HN32–HN35) that contained high amounts of sand and were completely barren of any foraminifera. Among the dominant taxa (> 10 % in at least 2 samples) were Ammonia sp., Quinqueloculina seminulum (Linnaeus, 1758), Quinqueloculina jugosa Cushman, 1944, Miliammina fusca (Brady, 1870), and Trochammina hadai Uchio, 1962. Accessory species (5 %–10 % in at least 2 samples) included Elphidium clavatum Cushman, 1930, Elphidium williamsoni Haynes, 1973, Elphidium oceanense (d'Orbigny, 1826), and Haynesina germanica (Ehrenberg, 1840). Among rare species (< 5 %), Ammoscalaria runiana (Heron-Allen and Earland, 1916), Elphidium albiumbilicatum (Weiss, 1954), Elphidium selseyense (Heron-Allen and Earland, 1911), and Miliolinella subrotunda (Montagu, 1803) were found (Plate 2). Although we did not observe elevated abundances of abnormal foraminiferal shells, shells of some species (mostly Ammonia sp.) showed various stages of carbonate dissolution.
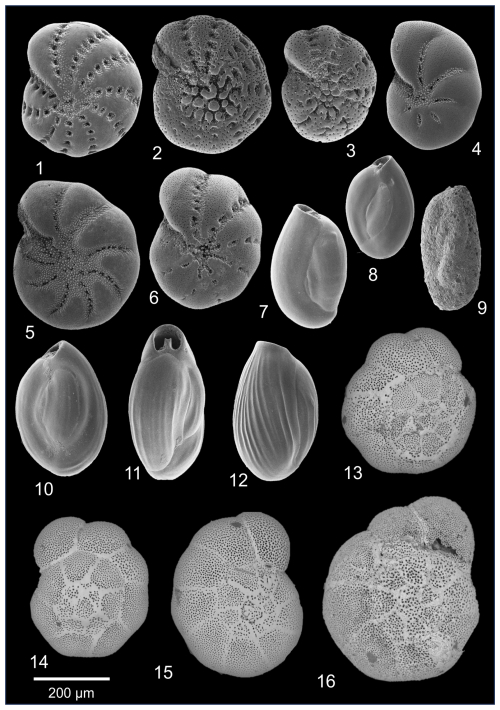
Plate 2Other most common benthic foraminiferal species from Hinsholmskilen harbour. (1) Elphidium williamsoni, (2–3) Elphidium oceanense, (4) Haynesina germanica, (5) Elphidium albiumbilicatum, (6) Elphidium clavatum, (7–8) Quinqueloculina seminulum, (9) Miliammina fusca, (10–12) Quinqueloculina jugosa. (13–16) Ammonia species showing different stages of dissolution: without dissolution traces (14) and with growing evidence of dissolution (13, 15–16).
The putatively invasive species Trochammina hadai (Fig. 5b) occurred in 16 out of 23 examined samples. The absolute abundance of T. hadai varied between 2.3 and 64.1 individuals per gram dry sediment (19.2 ind. g−1 on average), and its contribution to the foraminiferal assemblages constituted 1.3 % to 22.6 % (6.3 % on average). Stations with the highest numbers of T. hadai were HN12 and HN36 (20.4 % and 15.2 %, respectively) in the mid- and outer harbour, while the species was completely absent at stations HN25 and HN28 in the innermost harbour, at which other foraminiferal species prevailing in the assemblages were found.
Ammonia sp. occurred at all studied stations, where it always exceeded 15 % (range of 15.2 % to 81.5 %, with 53.2 % on average). Ammonia sp. was much more abundant than T. hadai and had absolute abundances ranging from 38.7 to 276.6 ind. g−1 (160 ind. g−1 on average). Selected specimens of Ammonia sp. were imaged with SEM and measured with the semi-automated method for pore diameter (Petersen et al., 2016) to distinguish morphologically the three Ammonia species which could potentially live in harbours: Ammonia confertitesta (phylotype T6), Ammonia aberdoveyensis (phylotype T2), and Ammonia veneta (phylotype T1). In total, 101 specimens were measured, and all had pore values ≥ 1.55 µm, which is above the pore threshold (1.4 µm) for Ammonia aberdoveyensis (phylotype T2) as defined by Richirt et al. (2019, 2021). The criterion to distinguish A. confertitesta (T6) morphologically from A. veneta (T1) is raised sutures in the latter. However, most Ammonia tests from 2019 sampling were slightly to badly dissolved (Plate 2: 13, 15–16; Supplement S7), and shell dissolution influences the size of pores and the raised sutures, which are the two main criteria to morphologically discriminate between these three Ammonia species. Therefore, due to the bad general preservation of Ammonia tests, we decided to keep the morphological identification at the genus level as Ammonia sp.
4.3 Morphological description of Trochammina hadai and Ammonia confertitesta
Morphologically, the Trochammina hadai specimens collected in Hinsholmskilen harbour compare favourably with the 11 paratypes of Trochammina hadai Uchio (1962) housed in the Cushman Collection (USNM CC 64882) at the Smithsonian National Museum of Natural History in Washington, D.C., USA. They are characterized by agglutinated tests that are white to yellowish brown in colour and are composed of fine-grained sand with occasional other lithic fragments (Plate 1, specimen 22013–22020). The tests are trochospiral, with 4.5 to 5 chambers per whorl, arranged in three to four whorls (Plate 1, specimen 1–2). The chambers are subglobular and inflate quickly, particularly in the last two whorls (Plate 1, specimen 5–6). All chambers may be seen on the dorsal side, but only those of the last whorl can be seen on the ventral side. The dorsal side is flat to slightly convex, and the ventral side is deeply umbilicate. The sutures are generally radial on both sides, and the aperture is an arched slit at the base of the ultimate chamber on the umbilical side (Plate 1, specimen 3, 4 and 8). Agglutinated tests of T. hadai did not react with 2 % HCl, suggesting organic cement, which is consistent with previous studies (e.g. Tremblin et al., 2021).
Specimens of Ammonia from Hinsholmskilen harbour were very similar to A. confertitesta individuals described in the extensive review on Ammonia species (Hayward et al., 2021: plate 26, specimens 13–15). This species is mainly described as unornamented and characterized by two to three whorls with seven to nine chambers in the last whorl, and this makes it difficult to discriminate it from other highly similar and unornamented Ammonia species observed in the northeastern Atlantic, i.e. A. aberdoveyensis and A. veneta. Also, due to slight to severe traces of dissolution in most Ammonia specimens in this study, they could not be identified at the species level solely based on morphological criteria. Therefore, these individuals were referred to as Ammonia sp. in the total assemblage dataset.
4.4 Molecular identification of Trochammina hadai and Ammonia confertitesta
The phylogenetic tree for Trochammina hadai (Fig. 2) contains 33 sequences of agglutinated foraminifera and is rooted in Reophacidae (R. scorpiurus, R. spiculifer, R. curtus, R. pilulifera). The obtained sequences cluster with T. hadai, supported by a high bootstrap value (BV) of 100 %. Trochammina hadai is part of a clade that contains Srinivasania sundarbanensis, Eggerelloides scaber, and Trochammina pacifica branching at the base. The clade, however, is not supported by a bootstrap value (BV). Two other clades are present in the tree. A second clade, also not sustained by a BV, consists of Bigenerina sp., Textularia gramen, Textularia agglutinans, Siphoniferoides sp., and Cyrea spp. branching as sister to the first clade. Liebusella goesi, Trochammina inflata, Trochammina sp., and Spiroplectammina sp. branch at the base of the two clades. A third clade without BV support contains Balticammina pseudomacrescens, Haplophragmoides wilberti, Entzia spp., and Arenoparrella mexicana.
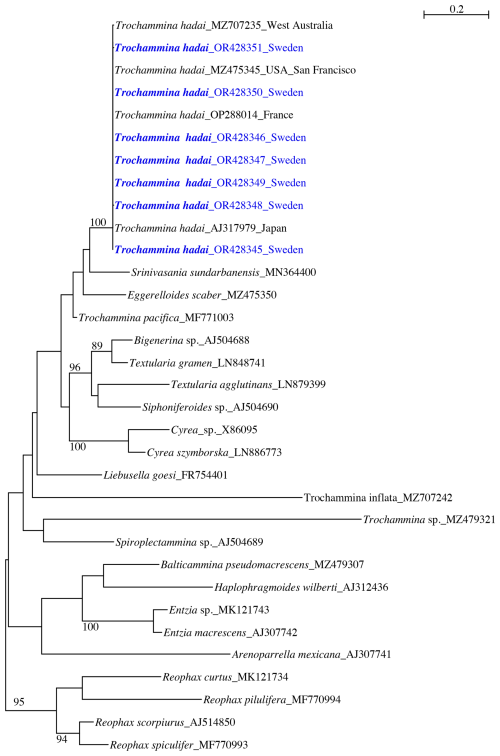
Figure 2Phylogenetic trees showing individuals of Trochammina hadai from Hinsholmskilen harbour plotted versus individuals reported and extracted to date from other places in the world. PhyML phylogenetic tree based on the 3'end fragment of the SSU rRNA gene, showing the evolutionary relationships of 33 foraminiferal sequences belonging to textulariids and Reophacidae. Specimens marked in blue indicate those for which sequences were acquired for the present study. The tree is rooted in Reophacidae (R. curtus, R. pilulifera, R. scorpiurus, and R. spiculifer). Specimens are identified by their accession numbers. Numbers at nodes indicate bootstrap values (BVs). Only BVs > 70 % are shown.
A total of 29 Ammonia specimens collected for DNA extractions were first identified morphologically. By examining their SEM images (Supplement S4) using the method described by Petersen et al. (2016), they were all morphologically identified as A. confertitesta with a pore diameter range of 1.8 to 4.3 µm. DNA barcoding agreed with this morphological identification based on SEM image morphometrics (Supplement S7).
4.5 Sediment analysis for potentially toxic elements
Several metals (Cd, Co, Ni, and Pb; Figs. 3–4) were distributed all over the harbour, falling mainly within high and good ecological quality status (EcoQS) corresponding to no or little deviation from reference conditions (Naturvårdsverket, 2000). Lead levels in proximity of the HP1 cleaning plant were slightly elevated and corresponded to intermediate EcoQS (Fig. 4b). Arsenic showed mainly high to good EcoQS but had its highest concentrations within the intermediate to poor EcoQS classes (a distinct to a large deviation) at a few locations scattered in the outer and the inner harbour in proximity of the HP1 cleaning plant (Fig. 5a). Both Zn and Cr had a clear distribution pattern, with mostly high to intermediate EcoQS in the outer harbour and poor to bad EcoQS for a major part of the inner harbour (Figs. 5b, 6a). Mercury had mostly moderate EcoQS but a few stations where EcoQS was poor (Fig. 6b). Finally, Cu was the only metal showing concentrations consistently falling within poor and bad EcoQS (a large to a very large deviation from reference conditions, respectively) all over the studied harbour (Fig. 7).
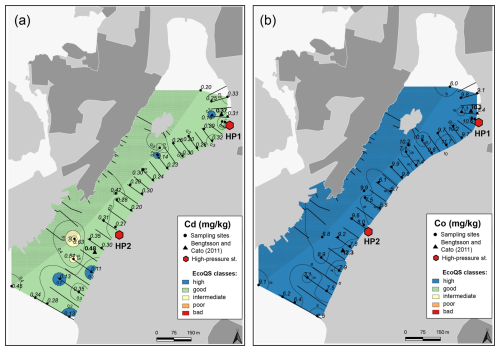
Figure 3Map showing the distribution of cadmium (a) and cobalt (b) in surface sediments of Hinsholmskilen harbour. Different colours or metal concentrations correspond to the five EcoQS classes as defined by the Swedish Environmental Protection Agency (Naturvårdsverket, 2000). HP1 and HP2 stand for high-pressure cleaning stations, where boats are lifted and rinsed with pressurized water.
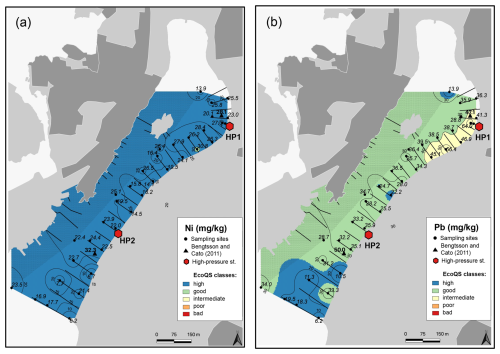
Figure 4Map showing the distribution of nickel (a) and lead (b) in surface sediments of Hinsholmskilen harbour. Different colours or metal concentrations correspond to the five EcoQS classes as defined by the Swedish Environmental Protection Agency (Naturvårdsverket, 2000). HP1 and HP2 stand for high-pressure cleaning stations, where boats are lifted and rinsed with pressurized water.
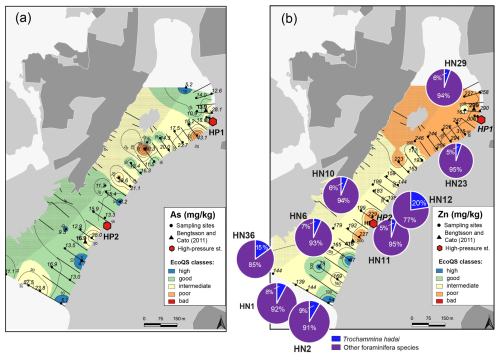
Figure 5Map showing the distribution of arsenic (a) and zinc together with the distribution of Trochammina hadai Uchio, 1962 versus other foraminiferal species (b) in surface sediments of Hinsholmskilen harbour. Different colours or concentrations correspond to the five EcoQS classes as defined by the Swedish Environmental Protection Agency (Naturvårdsverket, 2000). HP1 and HP2 stand for high-pressure cleaning stations, where boats are lifted and rinsed with pressurized water.
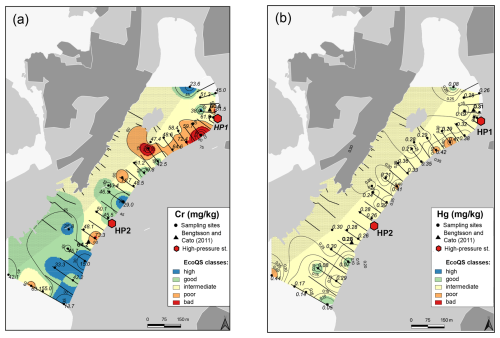
Figure 6Map showing the distribution of chromium (a) and mercury (b) in surface sediments of Hinsholmskilen harbour. Different colours or metal concentrations correspond to the five EcoQS classes as defined by the Swedish Environmental Protection Agency (Naturvårdsverket, 2000). HP1 and HP2 stand for high-pressure cleaning stations, where boats are lifted and rinsed with pressurized water.
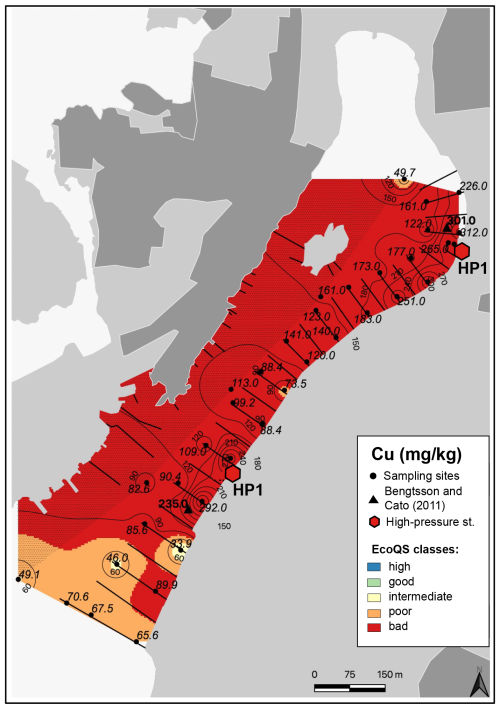
Figure 7Map showing the distribution of copper in surface sediments of Hinsholmskilen harbour. Different colours or metal concentrations correspond to the five EcoQS classes as defined by the Swedish Environmental Protection Agency (Naturvårdsverket, 2000). HP1 and HP2 stand for high-pressure cleaning stations, where boats are lifted and rinsed with pressurized water.
4.6 Statistical analysis
The RDA analysis using Cu, Zn, Co, As, Hg, Cd, and water depth was significant (p<0.05) and explained 29 % of the variability in the foraminiferal species abundances in Hinsholmen (Fig. 8). Only the first axis (RDA1; p<0.05) and both water depth (p<0.01) and Hg (p<0.05) were significant. Trochammina hadai was positively influenced by the water depth, as opposed to all the Elphidium species, which were negatively influenced by the same environmental parameter. The central position of Ammonia sp., H. germanica, Q. jugosa, and Q. seminulum on the first axis of the analysis indicated that the water depth had neither a positive nor a negative influence on these taxa. On the other hand, Ammonia sp. and H. germanica abundances were positively influenced by the Hg concentration, while both Quinqueloculina species and, to a certain extent, M. fusca were negatively influenced by this parameter. All the other potentially toxic elements analysed during this study had seemingly no impact on the other species, suggesting an influence of unanalysed environmental parameters.
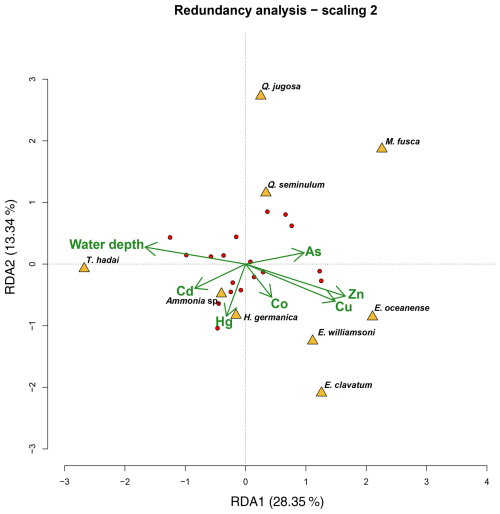
Figure 8Redundancy analysis of foraminiferal species and environmental data from the sampling campaign in 2019. The triplot (green) shows the constraining environmental parameters, while yellow triangles and red dots represent species data and sampling sites, respectively. The first and second axes (RDA1 and RDA2) explained 28.35 % and 13 % of the explained variability in the species, respectively.
5.1 Hinsholmskilen harbour: a new home for putatively invasive protists?
Based on dead and live benthic foraminiferal assemblage data, the foraminifera species reported herein represent a typical estuarine community with highly abundant Ammonia and Elphidium species, as documented elsewhere in European estuaries (among many others, Nikulina et al., 2008; Polovodova et al., 2009; Korsun et al., 2014; Darling et al., 2016; Bird et al., 2020; Fouet et al., 2022; Golikova et al., 2020; Jorissen et al., 2023; Pavard et al., 2023a, b).
Trochammina individuals found in Hinsholmskilen harbour clearly stand out compared to other Trochammina species previously recorded in Sweden by their large shells with inflated chambers and a shallow (1–2 m water depth) habitat. In Gullmar fjord, 12 species of Trochammina were reported by Höglund (1947), but none of these occur in such shallow-water conditions as those prevailing in Hinsholmskilen harbour. Despite a comprehensive body of literature on recent foraminiferal distribution in Swedish fjords and shallow inlets (e.g. Höglund, 1947; Gustafsson and Nordberg, 1999, 2000, 2001; Nordberg et al., 2017; Charrieau et al., 2018; Groeneveld et al., 2018; Choquel et al., 2021; Brinkmann et al., 2023; Morin et al., 2023; O'Brien et al., 2024), sediments in Swedish shallow leisure boat harbours and marinas have remained surprisingly understudied with respect to their benthic foraminiferal communities. Of the few studies performed to date, only Trochammina inflata was reported within the intertidal zone and up to 6 m water depth south and north of the city of Gothenburg (Alve and Murray, 1999; Murray and Alve, 1999). Foraminiferal communities in Gullmar fjord and the Skagerrak were also studied in the 1940s by Höglund (1947), who did not find T. hadai among other Trochammina species in shallow locations (7–10 m). However, seven specimens of Trochammina were collected in 2015 in a mudflat in Fiskebäckskil, Gullmar fjord (Supplement S6), and one of them (GF269, Table 1) could be successfully sequenced (GenBank accession number: PQ514028) and was identified as T. hadai. This species was also spotted in sedimentary eDNA from different water depths (30–117 m) of the Gullmar, Koljö, and Havstens fjords between 2017 and 2020, but whether this species was present there as dormant propagules (i.e. resting stages of foraminifera), waiting for optimal environmental conditions for growth, or was actually living in these fjords has not yet been verified by morphological data or single-cell DNA extractions (Brinkmann et al., 2023). Propagules can remain dormant up to 2 years and are often subject to transport by currents facilitating the spread and colonization of species (Alve and Goldstein, 2010).
Trochammina hadai Uchio, 1962 is a well-known putatively invasive species, with a native range of distribution in Asia, including Japan (Matoba, 1970; Matsushita and Kitazato, 1990), India (Rao, 1974; Rao et al., 2000), South Korea (Choi and An, 2012), and China (Zheng and Fu, 1990). It was introduced to the west coast of the United States sometime prior to 1971 (McGann, 2012), where it has outcompeted native foraminifera species (McGann, 2012) and is presently found in estuaries and harbours from Prince William Sound, Alaska, in the north to San Diego Bay, California, in the south (McGann et al., 2019). This species has expanded its range further by recently being recorded in Brazil (Eichler et al., 2018) and Australia (Tremblin et al., 2021) and has even arrived in Europe, with its first published record in the English Channel (Bouchet et al., 2023). Trochammina hadai is a shallow-water agglutinated species, thriving in brackish lakes, estuaries, harbours, and sheltered bays (Kitazato and Matsushita, 1996). The species is known by its faster reproduction, as compared to other Trochammina species (6 h vs. 24 h in T. inflata: Angell, 1990), and has a biphasic life cycle, with sexual reproduction in spring and asexual reproduction in autumn (Matsushita and Kitazato, 1990; Kitazato and Matsushita, 1996). A biphasic life cycle and the presence of dormant propagules are important for successful microorganism invasions (Ruiz et al., 2000). Sexual reproduction, releasing free-swimming gametes, is advantageous to maintain widespread distribution of T. hadai during seasonal oxygen depletion (Kitazato and Matsushita, 1996) and likely explains its invasive character and fast spreading rates. Choi and An (2012) previously reported an increased abundance of T. hadai in the inner Busan North Port (South Korea) polluted by Cu, Zn, and Pb but noted that T. hadai abundance decreased at the highest metal concentrations, which is consistent with the pattern observed in Hinsholmskilen harbour. This suggests species tolerance up to a certain metal concentration threshold.
Another well-known non-indigenous species, which has also been observed in Hinsholmskilen harbour, is Ammonia confertitesta Zheng, 1978 (phylotype T6). The species is known to have disjunct occurrences in Asia, Europe, and North America and belongs to a so-called “Ammonia tepida morphogroup”, which includes A. aberdoveyensis, A. confertitesta, and A. veneta (sensu Hayward et al., 2021). Having its type locality in China, A. confertitesta was suggested to have arrived in Europe from Asia (Pawlowski and Holzmann, 2008; Schweizer et al., 2011), and it has been reported ever since in coastal regions of France, the UK, the Netherlands, Germany, and Denmark (e.g. Schweizer et al., 2011; Saad and Wade, 2016; Bird et al., 2020; Francescangeli et al., 2021; Richirt et al., 2021; Pavard et al., 2023a, b). In Sweden, the presence of A. confertitesta was reported based on morphological and molecular data for Hanö Bay in the Baltic Sea (18 specimens; Groeneveld et al., 2018), Havstens Fjord (1 specimen; Brinkmann et al., 2023), and a mudflat in Fiskebäckskil in Gullmar fjord (38 specimens; Magali Schweizer, personal communication, 2024). In addition, high amounts of eDNA (40 % of the total number of foraminiferal reads) were identified for this species in the Gullmar, Koljö, and Havstens fjords, but, as the reads were mostly found in the 0.5 g sediment DNA extractions, they could mainly belong to propagules (Brinkmann et al., 2023).
Morphological identification of Ammonia individuals to the species level was difficult due to the dissolution of tests. Dissolution features similar to those observed in our study were reported from other shallow settings on the Swedish west coast, where calcareous species, abundant during the summer season, would suffer severe carbonate dissolution or even completely disappear in the autumn, leaving only inner organic linings behind due to severe seasonal hypoxia (Nordberg et al., 2017). In anoxic sediments, the pore water pH can drop to 6.9 (Ben-Yaakov, 1973), whereas significant dissolution in Ammonia species in culture experiments has been observed at pH levels between 7.1 and 7.9 (Haynert et al., 2011). In a study addressing the combined effects of deoxygenation, ocean acidification, and warming, Bernhard et al. (2021) found that propagule-grown assemblage changes were mainly driven by oxygen, whilst changes in temperature and pCO2 appeared of less importance to foraminifera. Strong carbonate dissolution in foraminiferal shells in estuaries was also attributed to cable bacteria activity, which significantly impacts sediment geochemistry and is associated with an “oxygen decline in the surface sediment combined with a pH maximum in the oxic zone, followed by a strong acidification of the pore water in the suboxic zone” (Daviray et al., 2024). Whether Hinsholmskilen harbour sediments had cable bacteria present or not is difficult to speculate, but we suggest that shell dissolution observed in our study likely occurred postmortem and resulted from the heavily anoxic layer below the sediment surface noted during sampling.
Molecular analysis confirmed that A. confertitesta was present in Hinsholmskilen harbour. Ammonia confertitesta is sensitive to anoxia and responds to severe oxygen depletion by dormancy reflected in reduced metabolism and low diatom feeding rates (LeKieffre et al., 2017). The species also shows cellular changes in the form of accumulated lipid droplets, an increase in electron-dense bodies, and possible cytosol metabolization as a response to environmental stress (Koho et al., 2018). This species neither stores intracellular NO nor shows any denitrification activity (Piña-Ochoa et al., 2010) and does not have a kleptoplastic activity either (Jauffrais et al., 2016, 2018). Most recent studies, however, show that A. confertitesta accumulates large quantities of intracellular phosphate with important implications for nutrient regulation and mitigation of eutrophication (Glock et al., 2025). Ammonia confertitesta is an effective heterotrophic omnivore, which seems opportunistic and feeds on organic detritus, bacteria, diatoms and other microalgae, fungi, and even small metazoans such as nematodes (Chronopoulou et al., 2019; Schweizer et al., 2022). In the studies of LeKieffre et al. (2017), Koho et al. (2018), and Piña-Ochoa et al. (2010), the Ammonia specimens were identified as Ammonia tepida or Ammonia sp., but molecular analyses on the same populations showed that the main phylotype was T6 (Bird et al., 2020), now known as A. confertitesta (Hayward et al., 2021). Ammonia confertitesta appears to have comparable ecological requirements to A. aberdoveyensis and A. veneta (Fouet et al., 2024), although the former may be more tolerant to brackish waters (Schweizer et al., 2011) and is more opportunistic (Fouet et al., 2024). The opportunistic behaviour of A. confertitesta can explain its preferential presence in highly industrialized harbours of the Elbe estuary and the English Channel, which have high metal pollution (Francescangeli et al., 2016; Pavard et al., 2023a, b), which is consistent with the positive relationship between Ammonia species and some of the metals in our study (Fig. 8). Another element which could help A. confertitesta to spread quickly is the high number of propagules it may produce, as two studies comparing morphological and eDNA data indicated a high percentage of reads for this species: 40 % of the total foraminiferal reads (Brinkmann et al., 2023) and 53 % of the Ammonia reads (Fouet et al., 2024), respectively.
The presence of two non-indigenous species raises a question about their vector of transport. The Port of Gothenburg is the largest port in Scandinavia, receiving about 5800 ship calls per year and about 30 % of Swedish foreign trade passing through it (Port of Gothenburg, 2023). The port is more than 400 years old and has a long history of international trade with Asia through the Swedish East India Company, which was founded in Gothenburg in 1731 and conducted 132 expeditions to China, trading tea, spices, porcelain, and silk. Hence, the ship traffic with its ballast water and sediment would be the most likely primary vector of transport for our two alien species. Since both T. hadai and A. confertitesta have recently been reported in French harbours of the English Channel (Bouchet et al., 2023; Pavard, 2023a, b), and given the prevailing current pattern with water transport through the English Channel into the Skagerrak and the Kattegat (Fig. 1a), the transport of both T. hadai and A. confertitesta to the Swedish west coast appears very likely. There are documented cases of other species introductions first appearing in France and then in Sweden, for instance, the Asian brush-clawed shore crab Hemigrapsus takanoi Asakura and Watanabe, 2005 and the Japanese shore crab Hemigrapsus sanguineus (de Haan, 1853), which both first arrived to Le Havre (France) in the late 1990s and since then spread along the NW European coasts from western France to the German North Sea, Wales and Scotland, eastern Ireland, western Sweden, and southern Norway (Karlsson et al., 2019). Also, both T. hadai and A. confertitesta have previously been suggested to originate from Asia, which is supported by many other species introduced to Sweden from Asia, as registered by the Swedish Agency for Marine and Water Management (SAMWM, 2023). Examples of those, among many others, are the toxic algae Pseudochattonella verruculosa Hara and Chinara, 1994 and Fibrocapsa japonica Toriumi and Takano, 1973, causing fish kills (Skjevik, 2004; Tango et al., 2004); the Japanese skeleton shrimp Caprella mutica Schurin, 1935, quickly reproducing and causing decline of the native shrimp Caprella linearis (Daneliya and Laakkonen, 2012); the diatom Coscinodiscus wailesii Gran and Angst 1931, causing massive algal blooms but not suitable as food for the native copepods (Jansen, 2008); the epiphytic red alga Bonnemaisonia hamifera Hariot 1891, creating dense carpets on hard bottoms but non-grazeable for herbivores due to its complex chemical defence mechanism (Enge et al., 2012); and, finally, the parasitic oyster mud worm Polydora websteri Hartman, 1943 (Loosanoff and Engle, 1943), likely introduced to Sweden together with the Japanese oyster Magallana gigas (Thunberg, 1793) and since then causing substantial problems in commercial oyster farming (Swedish Environmental Institute, 2020). The introduction of the Japanese oyster M. gigas may have facilitated the global spread of Trochammina hadai, as suggested in a review by McGann et al. (2025) (this volume).
In addition to transport by propagules, transport by leisure boat anchors appears a very likely secondary spreading vector (e.g. Simkanin et al., 2009; Ashton et al., 2022; Costello et al., 2022) for T. hadai and A. confertitesta, given the proximity of Hinsholmskilen harbour to the Port of Gothenburg. Both species could also arrive first in ballast water and sediment to the latter with larger commercial ships and then spread to the south with small boats, given their high numbers and numerous leisure boat harbours present in the region. At this point, it is impossible to speculate about the arrival times of T. hadai and A. confertitesta, since this study only provides a first glimpse of the composition of foraminiferal communities in Swedish leisure harbours mainly due to (a) lack of studies in the area and (b) former lumping of the “Ammonia group” morphology as Ammonia spp. in Swedish waters (e.g. Nordberg et al., 2017; Charrieau et al., 2018). Hence, future studies using sediment cores with SEM identification and sedaDNA analysis from the study area could help to unveil the history of introduction of both T. hadai and A. confertitesta to the region.
Regarding the potential ecological effects of both alien species in Hinsholmskilen harbour, T. hadai is not yet highly abundant (5 %–20 %) there; hence, ecological effects of its introduction and whether it will establish itself as an invasive species in the area remains to be seen. In contrast, based on morphological data, Ammonia species are highly dominant in the harbour today and comprise up to 81 % of the community, with molecular data suggesting the presence of mostly A. confertitesta (Supplement S7, see T6). Given that A. confertitesta has the highest known storage to date of intracellular phosphate among foraminifera, its potential ecological effects start to emerge and can be linked to the mitigation of coastal eutrophication (Glock et al., 2025). Future studies focused on living benthic foraminiferal communities in Hinsholmskilen harbour could establish whether A. confertitesta and T. hadai can outcompete native species with negative consequences for benthic diversity in the area.
5.2 Hinsholmskilen: small leisure harbour with high metal and arsenic pollution
Our results confirm that small boat harbours can be highly contaminated by metals and arsenic. Based on our study, most metal concentrations showed moderate to poor and bad EcoQS in the inner harbour and high to good EcoQS in the outer harbour. The only metals with different distributions were Hg and Cu, both of which showed poor and bad EcoQS, respectively, all over Hinsholmskilen harbour. This is consistent with a previous study by Bengtsson and Cato (2011), who investigated two sites in the inner and outer harbour in 2010 and found similar levels of metal in the surface sediments (Figs. 3–7).
The harbour is surrounded by exposed bedrock consisting of granodiorite and tonalite, so it is reasonable to assume that high metal concentrations in Hinsholmskilen can result from local bedrock. However, metal content in acid-leached granodiorite showed that bedrock levels for As, Cd, Co, Cu, Hg, Ni, Pb, and Zn did not exceed our levels, corresponding to high and good EcoQS (Dattola et al., 2024, and Table 3 therein). For tonalite, soils in the tonalite–trondjhemite series (central India) were shown to contain somewhat elevated Pb and Ni levels, equivalent to our levels of intermediate and poor EcoQS (Shukla et al., 2017). In our case, however, Ni has consistently low values (high EcoQS) all over the harbour, whilst Pb increases to intermediate EcoQS but only in the inner part close to the cleaning station HP1. Hence, because the general distribution pattern of metals and As matches the location of cleaning plants and because contaminant levels are generally higher in the inner harbour with higher boat traffic, we believe that boating activities and maintenance are the source of metals rather than local geology.
If, then, the highest metal pollution would be expected from boat traffic and maintenance, this should be reflected in the distribution of metals and metalloids specifically linked to boating activity, such as Co, Cu, Zn, Cr, Hg, Pb, and As. For instance, Co occurs in fossil fuels (Cato, 1997) and in this study has the highest concentrations proximal to the cleaning plants (Fig. 3b), which most boats pass through. Cobalt pollution is often linked to exhaust gases from boats and, in particular, to the old low-efficiency two-stroke engines most commonly used by small leisure boat owners. In these engines, up to 30 % of the fuel is directly discharged into the water un-combusted (Naturvårdsverket, 2009). Even though Co concentrations in Hinsholmskilen harbour remain at natural background level, the pattern of Co spatial distribution suggests an anthropogenic source due to the highest concentrations found close to the cleaning plants (Fig. 3b).
Copper showed a similar distribution pattern to Co, but, in contrast, Cu levels indicated bad EcoQS classes all over the harbour (Fig. 7). This metal is extensively used in antifouling paints for small leisure boats, and, in Sweden, Cu and Zn are the only biocides currently allowed in leisure boat paints (Cato, 1997; Thomas and Brooks, 2010; Nordberg et al., 2012; Moksnes et al., 2019). Copper enters the marine environment either during high boat season through leaching from boats or as “antifouling paint particles” (Turner, 2010) at high-pressure cleaning plants when boats are lifted on land, rinsed, and prepared for winter storage.
As, Zn, Cr, Hg, and Ni are also present in antifouling paints (Cato, 1997; Turner, 2010; Nordberg et al., 2012), and their levels increase again towards the inner harbour, reaching intermediate and poor EcoQS classes (Figs. 4a, 5a, 6a, b), which suggests small leisure boats with old paints as a possible impact source. In particular, Cr, Ni, and Pb are known to leach more rapidly from the antifouling paint particles than from the painted surface of the boats, which is caused by a greater surface area of pigments and additives exposed to a liquid medium (Turner, 2010). Also, in wooden docks, chromated copper arsenate is commonly used as an impregnating agent, with a lifespan of about 15 years (Hingston et al., 2001, and references therein). Studies on pollution caused by Cr, Cu, and As from wood impregnation have shown that the highest metal and As leakage from impregnated wood to the marine environment occurs soon after dock installation (Morrell and Huffman, 2004). The docks in Hinsholmskilen harbour were installed between 1965 and 1975 and have most likely required major maintenance for the replacement of wooden parts since their first installation, which would be a possible source of new As, Cr, and Zn pollution. Mercury, on the other hand, is rather evenly distributed in the harbour (Fig. 6b), reflecting a regional decrease in Hg deposition due to the phasing out of industrial Hg use since the late 1970s to early 1980s (e.g. Polovodova Asteman et al., 2015; Moros et al., 2017).
Finally, Pb distribution in the surface sediments of Hinsholmskilen harbour shows generally good EcoQS (Fig. 4b), with the exception of the innermost harbour, where sites proximal to the cleaning plant HP1 show intermediate EcoQS, again likely associated with metal runoff from land. Lead has also been used as a biocide in antifouling paints, but it was banned in 1960s, when it was replaced by TBT (Egardt et al., 2017). Along the Swedish west coast, Pb levels have also been steadily decreasing following the ban of leaded gasoline in 1995 (Polovodova Asteman et al., 2015; Nordberg et al., 2017; Kankainen et al., 2023), which is consistent with the low Pb concentrations found in Hinsholmskilen harbour surface sediments.
Bengtsson and Cato (2011) previously suggested that higher metal levels in the inner Hinsholmskilen harbour result from continuous metal supply from land, due to the boat uptake and cleaning and other maintenance work done in the harbour. Analyses of sludge in the sewage, stormwater, and drainage wells near cleaning stations showed that levels of metals, TBT, and Irgarol exceed bad EcoQS class, and high contaminant concentrations in the sludge were similar to levels found in hazardous waste (Bengtsson and Wernersson, 2012). In our study, higher concentrations of Cu, Pb, and Zn in proximity to the point source (high-pressure cleaning plants) clearly support this and demonstrate that small leisure boats may cause significant environmental impact on the marine environment.
When comparing average metal concentrations in the surface sediments found in other harbours and fjords on the Swedish west coast, Hinsholmskilen harbour clearly stands out with its high levels of Cr, Cu, and Zn (Fig. 9). Concentrations for those metals even exceed those in the previously highly polluted harbours of Byfjord (Uddevalla harbour) and Idefjord, which are both areas highly affected by past industrial activities, such as shipbuilding and pulp and paper mills, respectively (e.g. Rosenberg, 1977; Polovodova Asteman et al., 2015). This could be explained by comparing contaminant levels present in surface sediments only, since most of the industrial discharges in other harbours have ceased since the 1990s and can nowadays be found deeper in sediment core layers deposited over the 1970s and 1980s (e.g. Polovodova Asteman et al., 2015), which were not targeted in our study. Levels of Zn found in Hinsholmskilen harbour, on the other hand, are very close to what has been reported for the Port of Gothenburg and the inner estuary of the Göta älv river (Brack et al., 2001), around which about 70 % of Swedish industries are located within a radius of 500 km. Two other small leisure boat harbours of Grebbestad and Fiskebäckskil (Fig. 9) show either comparable or even higher levels of Cu, Zn, Hg, and Cd than Hinsholmskilen. This shows that leisure boat harbours indeed experience elevated concentrations of potentially toxic elements, and more research on these coastal settings could improve the understanding of potential ecosystem impacts.
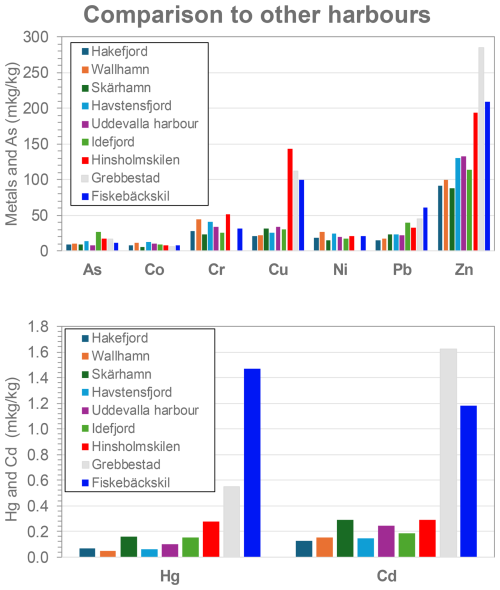
Figure 9Comparison of metal and arsenic distribution in surface sediments of Hinsholmskilen harbour (red) to other harbours and fjords on the Swedish west coast. Pollutant concentration data were averaged to obtain one mean value per harbour or fjord. Data for Hakefjorden, Wallhamn harbour, Skärhamn harbour, Havstens Fjord, Uddevalla harbour, and small boat harbours of Grebbestad and Fiskebäckskil represent previously unpublished data, while data for Idefjord are from O'Brien et al. (2024).
Based on our reconnaissance survey, the total foraminiferal assemblages in Hinsholmskilen harbour represent a typical estuarine community with highly abundant Ammonia and Elphidium species, as documented elsewhere in European estuaries. Both molecular and morphological data show the presence of two alien and putatively invasive species likely originating from Asia, Trochammina hadai and Ammonia confertitesta (phylotype T6), suggesting both propagules and transport by leisure boat anchors as very likely secondary spreading vectors. Both T. hadai and A. confertitesta could first have arrived in ballast water and sediment to the Port of Gothenburg with larger commercial ships, or with introduced oysters to the adjacent Scandinavian seas, and then spread further with small boats and propagules. Based on potential toxic element analysis, Hinsholmskilen harbour has good to high EcoQS corresponding to no or little deviation from reference conditions for Cd, Co, Ni, and Pb distribution. In contrast, Pb, As, Zn, and Cr all show poor to bad EcoQS in the innermost harbour in proximity to high-pressure cleaning plants, where leisure boats are usually lifted, cleaned, and prepared for winter storage on land. Finally, Cu and Hg show consistently bad and poor EcoQS all over the harbour, suggesting continuous use of both metals as biocides in antifouling boat paints. Our study shows that leisure boat harbours experience environmental problems related to pollution and the introduction of alien and invasive species, and additional research on these coastal settings could help further understand their potential ecosystem impacts.
The potentially toxic element, census foraminifera, and Ammonia pore measurement data are available in the Supplement (S1–S7) of this paper. All molecular data were deposited in the NCBI/GenBank database (https://www.ncbi.nlm.nih.gov/genbank/, National Library of Medicine, 2025). SEM pore images of Ammonia individuals from total fauna assemblages were too large to upload but can be provided on request.
Microslides with individuals of Ammonia confertitesta and Trochammina hadai are available at the Gothenburg Natural History Museum (Protista/Protozoa Catalogue: numbers GNM Prot. 93 and GNM Prot. 94, respectively).
The supplement related to this article is available online at https://doi.org/10.5194/jm-44-119-2025-supplement.
IPA, KN, and MS developed the idea and designed the project. EJ, AO, MH, MM, JCP, MS, DR, and IPA carried out the analyses and analysed the data. IPA and KN supervised the thesis work of students EJ and OA, respectively. All authors contributed to article writing.
The contact author has declared that none of the authors has any competing interests.
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This article is part of the special issue “Advances and challenges in modern and benthic foraminifera research: a special issue dedicated to Professor John Murray”. It is not associated with a conference.
Göteborgsregionens Fritidshamnar AB (GREFAB) provided information about Hinsholmskilen harbour and made their work boat available for sediment sampling. Amelie Sjösten, Lennart Bornmalm (Department of Marine Sciences, University of Gothenburg), and Roger Lindberg (GREFAB) assisted during sediment sampling. Henrik Bengtsson (Administration of Västra Götaland County) made heavy metal data from the study by Bengtsson and Cato (2011) available. Matthias Konrad-Schmolke and Filip Morin assisted with SEM imaging, while Alexander Sundestrand helped with GIS. Sophie Quinchard (LPG, Angers) helped with molecular analyses of Ammonia, while Geoffroy Couasnet (LPG, Angers) made a new macro available for measuring Ammonia pores based on the semi-automated method developed by Jassin Petersen. The authors are also grateful to the two reviewers and the editor for constructive comments and feedback on an earlier version of this paper. This paper is a contribution to a special JM issue dedicated to John W. Murray, who inspired multiple generations of foraminifera researchers worldwide, including the authors of this paper.
The molecular work on T. hadai was supported by a Schmidheiny Foundation grant (MH).
The publication of this article was funded by the Swedish Research Council, Forte, Formas, and Vinnova.
This paper was edited by Malcolm Hart and reviewed by Duncan Pirrie and one anonymous referee.
Alve, E. and Goldstein, S. T.: Dispersal, survival and delayed growth of benthic foraminiferal propagules, J. Sea Res., 63, 36–51, https://doi.org/10.1016/j.seares.2009.09.003, 2010.
Alve, E. and Murray, J. W.: Marginal marine environments of the Skagerrak and Kattegat: a baseline study of living (stained) benthic foraminiferal ecology, Palaeogeogr. Palaeocl., 146, 171–193, https://doi.org/10.1016/S0031-0182(98)00131-X, 1999.
Angell, R. W.: Observations on reproduction and juvenile test building in the foraminifer Trochammina inflata, J. Foramin. Res., 20, 246–247, https://doi.org/10.2113/gsjfr.20.3.246, 1990.
Arenas, F., Bishop, J. D. D., Carlton, J. T., Dyrynda, P. J., Farnham, W. F., Gonzalez, D. J., Jacobs, M. W., Lambert, C., Lambert, G., Nielsen, S. E., Pedersen, J. A., Porter, J. C., Ward, S., and Wood, C. A.: Alien species and other notable records from a rapid assessment survey of marinas on the south coast of England, J. Mar. Biol. Assoc. UK, 86, 1329–1337, 2006.
Asakura, A. and Watanabe, S.: Hemigrapsus takanoi, new species, a sibling species of the common Japanese intertidal Crab H. penicillatus (Decapoda: Brachyura: Grapsoidea), J. Crustacean Biol., 25, 279–292, 2005.
Ashton, G. V., Zabin, C. J., Davidson, I. C., and Ruiz, G. M.: Recreational boats routinely transfer organisms and promote marine bioinvasions, Biol. Invasions, 24, 1083–1096, https://doi.org/10.1007/s10530-021-02699-x, 2022.
Bax, N., Williamson, A., Aguero, M., Gonzalez, E., and Geeves, W.: Marine invasive alien species: a threat to global biodiversity, Mar. Policy, 27, 313–323, https://doi.org/10.1016/S0308-597X(03)00041-1, 2003
Bengtsson, H. and Cato, I.: TBT i småbåtshamnar i Västra Götalands Län 2010: En studie av belastning och trender, Länsstyrelsen i Västra Götalands län, Rapport nr 2011:30, 65 s. [TBT in small leisure boat harbours in the Västra Götaland County in 2010: a study of impact and trends, County Administrative Board of Västra Götaland, Report no. 2011:30, 65 pp.], https://naturvardsverket.diva-portal.org/smash/get/diva2:1345312/FULLTEXT01.pdf (last access: 15 May 2025), 2011 (in Swedish).
Bengtsson, H. and Wernersson, A.-S.: TBT, koppar, zink och irgarol i dagvatten, slam och mark i småbåtshamnar, Västra Götalands län 2011. Länsstyrelsen i Västra Götaland, Göteborg, Rapport nr 2012:16, 68 s. [TBT, copper, zinc and irgarol in storm water and sludge of drainage wells and soils in leisure boat harbours of Västra Götaland County in 2011, County Administrative Board of Västra Götaland, Report nr 2012:16, 68 pp.], https://naturvardsverket.diva-portal.org/smash/get/diva2:1345314/FULLTEXT01.pdf (last access: 15 May 2025) 2012 (in Swedish).
Ben-Yaakov, S.: pH Buffering of pore water of recent anoxic marine sediments 1, Limnol. Oceanogr., 18, 86–94, https://doi.org/10.4319/lo.1973.18.1.0086, 1973.
Bernhard, J. M., Wit, J. C., Starczak, V. R., Beaudoin, D. J., Phalen, W. G., and McCorkle, D. C.: Impacts of multiple stressors on a benthic foraminiferal community: A long-term experiment assessing response to ocean acidification, hypoxia and warming, Front. Mar. Sci., 8, 643339, https://doi.org/10.3389/fmars.2021.643339, 2021.
Bird, C., Schweizer, M., Roberts, A., Austin, W. E., Knudsen, K. L., Evans, K. M., Filipsson, H. L., Sayer, M. D. J., Geslin, E., and Darling, K. F.: The genetic diversity, morphology, biogeography, and taxonomic designations of Ammonia (Foraminifera) in the Northeast Atlantic, Mar. Micropaleontol., 155, 101726, https://doi.org/10.1016/j.marmicro.2019.02.001, 2020.
Bolam, S. G.: Impacts of dredged material disposal on macrobenthic invertebrate communities: a comparison of structural and functional (secondary production) changes at disposal sites around England and Wales, Mar. Pollut. Bull., 64, 2199–2210, https://doi.org/10.1016/j.marpolbul.2012.07.050, 2012.
Bouchet, V. M., Debenay, J. P., and Sauriau, P. G.: First report of Quinqueloculina carinatastriata (foraminifera) along the French Atlantic coast (Marennes-Oléron Bay and Ile de Ré), J. Foramin. Res., 37, 204–212, 2007.
Bouchet, V. M., Pavard, J. C., Holzmann, M., McGann, M., Arminot du Châtelet, E., Courleux, A., Pezy, J. P., Dauvin, J. C., and Seuront, L.: The invasive Asian benthic foraminifera Trochammina hadai Uchio, 1962: identification of a new local in Normandy (France) and a discussion on its putative introduction pathways, Aquat. Invasions, 18, 23–38, https://doi.org/10.3391/ai.2023.18.1.103512, 2023.
Brady, G. S. and Robertson, D.: XXVI. – The Ostracoda and Foraminifera of tidal rivers. With an analysis and descriptions of the Foraminifera, Brady, H. B. F. L. S., Ann. Mag. Nat. Hist., 6, 273–309, 1870.
Brack, K., Johannesson, L., and Stevens, R.: Accumulation rates and mass calculations of Zn and Hg in recent sediments, Göta älv estuary, Sweden, Environ. Geol., 40, 1232–1241, https://doi.org/10.1007/s002540100293, 2001.
Brennecke, D., Duarte, B., Paiva F., Caçador, I., and Canning-Clode J.: Microplastics as vector for heavy metal contamination from the marine environment, Estuar. Coast. Shelf S., 178, 189–195, https://doi.org/10.1016/j.ecss.2015.12.003, 2016.
Brinkmann, I., Schweizer, M., Singer, D., Quinchard, S., Barras, C., Bernhard, J. M., and Filipsson, H. L.: Through the eDNA looking glass: Responses of fjord benthic foraminiferal communities to contrasting environmental conditions, J. Eukaryot. Microbiol., 70, e12975, https://doi.org/10.1111/jeu.12975, 2023.
Calvo-Marcilese, L. and Langer, M. R.: Breaching biogeographic barriers: the invasion of Haynesina germanica (Foraminifera, Protista) in the Bahía Blanca estuary, Argentina, Biol. Invasions, 12, 3299–3306, https://doi.org/10.1007/s10530-010-9723-x, 2010.
Cato, I.: Sedimentundersökningar längs Bohuskusten 1995 samt nuvarande trender i kustsedimentensmiljökvalitet – en rapport från fem kontrollprogram [Investigation of sediments along the Swedish Bohuslän coast in 1995 and recent trends in sediment environmental quality – a report from five control programs], Geological Survey of Sweden (SGU), Research Reports 95, 193–266, 1997 (in Swedish).
Charrieau, L. M., Filipsson, H. L., Ljung, K., Chierici, M., Knudsen, K. L., and Kritzberg, E.: The effects of multiple stressors on the distribution of coastal benthic foraminifera: A case study from the Skagerrak-Baltic Sea region, Mar. Micropaleontol., 139, 42–56, https://doi.org/10.1016/j.marmicro.2017.11.004, 2018.
Choi, J. U. and An, S.: High benthic foraminiferal diversity in polluted Busan North Port (Korea), J. Foramin. Res., 42, 327–339, https://doi.org/10.2113/gsjfr.42.4.327, 2012.
Choquel, C., Geslin, E., Metzger, E., Filipsson, H. L., Risgaard-Petersen, N., Launeau, P., Giraud, M., Jauffrais, T., Jesus, B., and Mouret, A.: Denitrification by benthic foraminifera and their contribution to N-loss from a fjord environment, Biogeosciences, 18, 327–341, https://doi.org/10.5194/bg-18-327-2021, 2021.
Chronopoulou, P. M., Salonen, I., Bird, C., Reichart, G. J., and Koho, K. A.: Metabarcoding insights into the trophic behavior and identity of intertidal benthic foraminifera, Front. Microbiol., 10, 1169, https://doi.org/10.3389/fmicb.2019.01169, 2019.
Costello, K. E., Lynch, S. A., McAllen, R., O'Riordan, R. M., and Culloty, S. C.: Assessing the potential for invasive species introductions and secondary spread using vessel movements in maritime ports, Mar. Pollut. Bull., 177, 113496, https://doi.org/10.1016/j.marpolbul.2022.113496, 2022.
Cushman, J. A.: The Foraminifera of the Atlantic Ocean. Part 7. Nonionidae, Camerinidae, Peneroplidae and Alveolinellidae, Bull. US Nat. Museum, 104, 1–79, https://www.biodiversitylibrary.org/page/7879320 (last access: 15 May 2025), 1930.
Cushman, J. A.: Foraminifera from the shallow water of the New England coast, Special Publ. No. 12, Cushman Lab. Foram. Res., 37 pp., 1944.
Daneliya, M. E. and Laakkonen, H.: The Japanese skeleton shrimp Caprella mutica (Amphipoda: Caprellidae) in Sweden (Eastern Skagerrak), Mar. Biodivers. Records, 5, e36, https://doi.org/10.1017/S1755267212000243, 2012.
Danielssen, D. S., Edler, L., Fonselius, S., Hernroth, L., Ostrowski, M., Svendsen, E., and Talpsepp, L.: Oceanographic variability in the Skagerrak and northern Kattegat, May–June, 1990, ICES J. Mar. Sci., 54, 753–773, https://doi.org/10.1006/jmsc.1996.0210, 1997.
Darling, K. F., Schweizer, M., Knudsen, K. L., Evans, K. M., Bird, C., Roberts, A., Filipsson, H. L., Kim, J. H., Gudmundsson, G., Wade, C. M., Sayer, M. D. J., and Austin, W. E.: The genetic diversity, phylogeography and morphology of Elphidiidae (Foraminifera) in the Northeast Atlantic, Mar. Micropaleontol., 129, 1–23, https://doi.org/10.1016/j.marmicro.2016.09.001, 2016.
Dattola, L., Belvedere, A., D’Agostino, M., Faggio, G., Majolino, D., Marguccio, S., Messina, G., Messina, M., Mottese, A. F., Paladini, Venuti, V., and Caridi, F.: Assessment of the Radioactivity, Metals Content and Mineralogy of Granodiorite from Calabria, Southern Italy: A Case Study, Materials, 17, 3813, https://doi.org/10.3390/ma17153813, 2024.
Daviray, M., Geslin, E., Risgaard-Petersen, N., Scholz, V. V., Fouet, M., and Metzger, E.: Potential impacts of cable bacteria activity on hard-shelled benthic foraminifera: implications for their interpretation as bioindicators or paleoproxies, Biogeosciences, 21, 911–928, https://doi.org/10.5194/bg-21-911-2024, 2024.
de Haan, W.: Crustacea, in: Fauna Japonica sive Descriptio Animalium, quae in Itinere per Japoniam, Jussu et Auspiciis Superiorum, qui Summum in India Batava Imperium Tenent, Suspecto, Annis 1823–1830 Collegit, Notis, Observationibus et Adumbrationibus Illustravit. i–xxxi, ix–xvi, 1–243, pls. A–J, L–Q, 1–55, Lugduni-Batavorum, edited by: von Siebold, P. F., 58 pp., https://www.biodiversitylibrary.org/item/217507#page/41/mode/1up (last access: 20 May 2025), 1853.
Deldicq, N., Alve, E., Schweizer, M., Asteman, I. P., Hess, S., Darling, K., and Bouchet, V. M.: History of the introduction of a species resembling the benthic foraminifera Nonionella stella in the Oslofjord (Norway): morphological, molecular and paleo-ecological evidences, Aquat. Invasions, 14, 182–205, 2019.
Dennison, W. C., Orth, R. J., Moore, K.A., Stevenson, J .C., Carter, V., Kollar, S., Bergström, P. W., and Batiuk, R. A.: Assessing water quality with submerged aquatic vegetation: Habitat requirements as barometers of Chesapeake Bay health, BioScience, 43, 86–94, https://doi.org/10.2307/1311969, 1993.
Desprez, M.: Physical and biological impact of marine aggregate extraction along the French coast of the Eastern English Channel: short-and long-term post-dredging restoration, ICES J. Mar. Sci., 57, 1428–1438, https://doi.org/10.1006/jmsc.2000.0926, 2000.
d'Orbigny, A. D.: Tableau méthodique de la classe des Céphalopodes, Ann. Sci. Nat., 7, 96–169, 245–314, http://biodiversitylibrary.org/page/5753959 (last access: 15 May 2025), 1826.
Egardt, J., Nilsson, P., and Dahllöf, I.: Sediments indicate the continued use of banned antifouling compounds, Mar. Pollut. Bull., 125, 282–288, https://doi.org/10.1016/j.marpolbul.2017.08.035, 2017.
Ehrenberg, C. G.: Eine weitere Erläuterung des Organismus mehrerer in Berlin lebend beobachteter Polythalamien der Nordsee, Bericht über die zur Bekanntmachung geeigneten Verhandlungen der Königlich-Preussischen Akademie der Wissenschaften zu Berlin, 1840, 18–23, 1840.
Eichler, P. P., McGann, M., Rodrigues, A. R., Mendonca, A., Amorim, A., Bonetti, C., Cordeiro de Farias, C., Melo e Sousa, S. H., Vital, H., and Gomes, M. P.: The occurrence of the invasive foraminifera Trochammina hadai Uchio in Flamengo inlet, Ubatuba, São Paulo state, Brazil, Micropaleontology, 64, 391–402, https://www.jstor.org/stable/26759098 (last access: 17 May 2025), 2018.
Eklund, B., Hansson, T., Bengtsson, H., and Eriksson Wiklund, A. K.: Pollutant concentrations and toxic effects on the red alga Ceramium tenuicorne of sediments from natural harbours and small boat harbours on the west coast of Sweden, Arch. Environ. Con. Tox., 70, 583–594, https://doi.org/10.1007/s00244-016-0262-z, 2016.
Enge, S., Nylund, G. M., Harder, T., and Pavia, H.: An exotic chemical weapon explains low herbivore damage in an invasive alga, Ecology, 93, 2736–2745, https://doi.org/10.1890/12-0143.1, 2012.
Epstein, S. and López-García, P.: “Missing” protists: a molecular prospective, Biodivers. Conserv., 17, 261–276, https://doi.org/10.1007/s10531-007-9250-y, 2008.
Farrell, P. and Nelson, K.: Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.), Environ. Pollut., 177, 1–3, https://doi.org/10.1016/j.envpol.2013.01.046, 2013.
Ferrario, J., Caronni, S., Occhipinti-Ambrogi, A., and Marchini, A.: Role of commercial harbours and recreational marinas in the spread of non-indigenous fouling species, Biofouling, 33, 651-660, https://doi.org/10.1080/08927014.2017.1351958, 2017.
Fouet, M. P., Singer, D., Coynel, A., Héliot, S., Howa, H., Lalande, J., Mouret, A., Schweizer., M., Tcherkez, G., and Jorissen, F. J.: Foraminiferal distribution in two estuarine intertidal mudflats of the French Atlantic coast: testing the Marine Influence Index, Water, 14, 645, https://doi.org/10.3390/w14040645, 2022.
Fouet, M. P., Schweizer, M., Singer, D., Richirt, J., Quinchard, S. and Jorissen, F. J.: Unravelling the distribution of three Ammonia species (Foraminifera, Rhizaria) in French Atlantic Coast estuaries using morphological and metabarcoding approaches, Mar. Micropaleontol., 188, 102353, https://doi.org/10.1016/j.marmicro.2024.102353, 2024.
Francescangeli, F., Du Chatelet, E. A., Billon, G., Trentesaux, A., and Bouchet, V. M. P.: Palaeo-ecological quality status based on foraminifera of Boulogne-sur-Mer harbour (Pas-de-Calais, Northeastern France) over the last 200 years, Mar. Environ. Res., 117, 32–43, 2016.
Francescangeli, F., Milker, Y., Bunzel, D., Thomas, H., Norbisrath, M., Schönfeld, J., and Schmiedl, G.: Recent benthic foraminiferal distribution in the Elbe Estuary (North Sea, Germany): A response to environmental stressors, Estuar. Coast. Shelf S., 251, 107198, https://doi.org/10.1016/j.ecss.2021.107198, 2021.
Glock, N.: Benthic foraminifera and gromiids from oxygen-depleted environments – survival strategies, biogeochemistry and trophic interactions, Biogeosciences, 20, 3423–3447, https://doi.org/10.5194/bg-20-3423-2023, 2023.
Glock, N., Richirt, J., Woehle, C., Algar, C., Armstrong, M., Eichner, D., Firrincieli, H., Makabe, A., Menon, A. G., Ishitani, Y., Hackl, T., Hubert-Huard, R., Kienast, M., Milker, Y., Mutzberg, A., Ni, S., Okada, S., Rakshit, S., Schmiedl, G., Steiner, Z., Tame, A., Zhang, Z., and Nomaki, H.: Widespread occurrence and relevance of phosphate storage in foraminifera, Nature, 638, 1000–1006, https://doi.org/10.1038/s41586-024-08431-8, 2025.
Goetz, E. J., Yan, A., Hull, P. M., and Thomas, E.: Ammonia (Foraminifera) in Long Island Sound (USA): Molecular and Morphological Diversity, J. Foramin. Res., 55, 45–59, https://doi.org/10.61551/gsjfr.55.1.45, 2025
Golikova, E., Varfolomeeva, M., Yakovis, E., and Korsun, S.: Saltmarsh foraminifera in the subarctic White Sea: thrive in summer, endure in winter, Estuar. Coast. Shelf S., 238, 106685, https://doi.org/10.1016/j.ecss.2020.106685, 2020.
Gomaa, F., Utter, D. R., Powers, C., Beaudoin, D. J., Edgcomb, V. P., Filipsson, H. L., Hansel, C. M., Wankel, S. D., Zhang, Y., and Bernhard, J. M.: Multiple integrated metabolic strategies allow foraminiferan protists to thrive in anoxic marine sediments, Sci. Adv., 7, eabf1586, https://doi.org/10.1126/sciadv.abf1586, 2021.
Gouy, M., Guindon, S., and Gascuel, O.: SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building, Mol. Biol. Evol., 27, 221–224, https://doi.org/10.1093/molbev/msp259, 2010.
Grenfell, H. R., Hayward, B. W., and Horrocks, M.: Foraminiferal record of ecological impact of deforestation and oyster farms, Mahurangi Harbour, New Zealand, Mar. Freshwater Res., 58, 475–491, 2007.
Groeneveld, J., Filipsson, H. L., Austin, W. E., Darling, K., McCarthy, D., Quintana Krupinski, N. B., Bird, C. and Schweizer, M.: Assessing proxy signatures of temperature, salinity, and hypoxia in the Baltic Sea through foraminifera-based geochemistry and faunal assemblages, J. Micropalaeontol., 37, 403–429, https://doi.org/10.5194/jm-37-403-2018, 2018.
Guindon, S., Dufayard, J. F., Lefort, V., Anisimova, M., Hordijk, W., and Gascuel, O.: New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0, Syst. Biol., 59, 307–321, https://doi.org/10.1093/sysbio/syq010, 2010.
Gustafsson, M. and Nordberg, K.: Benthic foraminifera and their response to hydrography, periodic hypoxic conditions and primary production in the Koljö fjord on the Swedish west coast, J. Sea Res., 41, 163–178, https://doi.org/10.1016/S1385-1101(99)00002-7, 1999.
Gustafsson, M. and Nordberg, K.: Living (stained) benthic foraminifera and their response to the seasonal hydrographic cycle, periodic hypoxia and to primary production in Havstens Fjord on the Swedish west coast, Estuar. Coast. Shelf S., 51, 743–761, https://doi.org/10.1006/ecss.2000.0695, 2000.
Gustafsson, M. and Nordberg, K.: Living (stained) benthic foraminiferal response to primary production and hydrography in the deepest part of the Gullmar Fjord, Swedish West Coast, with comparisons to Hoglund's 1927 material, J. Foramin. Res., 31, 2–11, https://doi.org/10.2113/0310002, 2001.
Hara, Y., Doi, K., and Chihara, M.: Four new species of Chattonella (Raphidophyceae, Chromophyta) from Japan, Jpn. J. Phycol., 42, 407–420, 1994.
Harvey, M., Gauthier, D., and Munro, J.: Temporal changes in the composition and abundance of the macro-benthic invertebrate communities at dredged material disposal sites in the Anse à Beaufils, Baie des Chaleurs, Eastern Canada, Mar. Pollut. Bull., 36, 41–55, https://doi.org/10.1016/S0025-326X(98)90031-5, 1998.
Haynert, K., Schönfeld, J., Riebesell, U., and Polovodova, I.: Biometry and dissolution features of the benthic foraminifer Ammonia aomoriensis at high pCO2, Mar. Ecol. Prog. Ser., 432, 53–67, 2011.
Haynert, K., Schönfeld, J., Riebesell, U., and Polovodova, I.: Biometry and dissolution features of the benthic foraminifer Ammonia aomoriensis at high pCO2, Mar. Ecol. Prog. Ser., 432, 53–67, 2011.
Haynes, J. R.: Cardigan Bay Recent Foraminifera (Cruises of the R. V. Antur, 1962–1964), Bulletin of the British Museum (Natural History), Zoology, Supplement 4, 245 pp., https://biodiversitylibrary.org/page/44726681 (last access: 15 May 2025), 1973.
Hayward, B. W.: Introduced marine organisms in New Zealand and their impact in the Waitemata Harbour, Auckland, Tane, 36, 197–223, 1997.
Hayward, B. W., Holzmann, M., Grenfell, H. R., Pawlowski, J., and Triggs, C. M.: Morphological distinction in Ammonia-towards a taxonomic revision of the world's most commonly misidentified foraminifera, Mar. Micropaleontol., 50, 237–271, https://doi.org/10.1016/S0377-8398(03)00074-4, 2004.
Hayward, B. W., Holzmann, M., Pawlowski, J., Parker, J. H., Kaushik, T., Toyofuku, M. S., and Tsuchiya, M.: Molecular and morphological taxonomy of living Ammonia and related taxa (Foraminifera) and their biogeography, Micropaleontology, 67, 109–313, https://doi.org/10.47894/mpal.67.3.01, 2021.
Heron-Allen, E. and Earland, A.: On the recent and fossil Foraminifera of the shore-sands of Selsey Bill, Sussex – VIII. Tabular list of species and localities, Journal of the Royal Microscopical Society, 8, 436–448, https://www.biodiversitylibrary.org/page/2210414 (last access: 17 May 2025), 1911.
Heron-Allen, E. and Earland, A.: The Foraminifera of the West of Scotland. Collected by on the cruise of the S.Y. “Runa”, July–Sept. Being a contribution to “Spolia Runiana”, Transactions of the Linnean Society of London, Zoology, 11, 197–299, https://www.biodiversitylibrary.org/page/16393732 (last access: 15 May 2025), 1916.
Hingston, J. A., Collins, C. D., Murphy, R. J., and Lester, J. N.: Leaching of chromated copper arsenate wood preservatives: a review, Environ. Pollut., 111, 53–66, https://https://doi.org/10.1016/S0269-7491(00)00030-0, 2001.
Hinsholmens Båtklubb: http://www.hinsholmen.se, last access: 5 June 2023.
Hirai, H., Takada, H., Ogata, Y., Yamashita, R., Mizukawa, K., Saha, M., Kwan, C., Moore, C., Gray, H., Laursen, D., Zettler, E. R., Farrington, J. W., Reddy, C. M., Peacock, E. E., and Ward, M. W.: Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches, Mar. Pollut. Bull., 62, 1683–1692, https://doi.org/10.1016/j.marpolbul.2011.06.004, 2011.
Hyams-Kaphzan, O., Almogi-Labin, A., Sivan, D., and Benjamini, C.: Benthic foraminifera assemblage change along the southeastern Mediterranean inner shelf due to fall-off of Nile-derived siliciclastics, Neues Jahrb. Geol. P.-A., 248, 315–344, 2008.
Höglund, H.: Foraminifera in the Gullmar Fjord and the Skagerak. Uppsala University, Zoologiska Bidrag, 26, 1–328, 1947.
Hülsmann, N. and Galil, B. S.: Protists – a dominant component of the ballast-transported biota, in: Invasive aquatic species of Europe. Distribution, impacts and management, edited by: Leppäkoski, E., Gollasch, S., and Olenin, S., 20–26, Springer, Dordrecht, the Netherlands, 20–26, https://https://doi.org/10.1007/978-94-015-9956-6, 2002.
Jansen, S.: Copepods grazing on Coscinodiscus wailesii: a question of size? Helgoland, Mar. Res., 62, 251–255, https://doi.org/10.1007/s10152-008-0113-z, 2008.
Jauffrais, T., Jesus, B., Metzger, E., Mouget, J.-L., Jorissen, F., and Geslin, E.: Effect of light on photosynthetic efficiency of sequestered chloroplasts in intertidal benthic foraminifera (Haynesina germanica and Ammonia tepida), Biogeosciences, 13, 2715–2726, https://doi.org/10.5194/bg-13-2715-2016, 2016.
Jauffrais, T., LeKieffre, C., Koho, K. A., Tsuchiya, M., Schweizer, M., Bernhard, J. M., Meibom, A., and Geslin, E.: Ultrastructure and distribution of kleptoplasts in benthic foraminifera from shallow-water (photic) habitats, Mar. Micropaleontol., 138, 46–62, https://doi.org/10.1016/j.marmicro.2017.10.003, 2018.
Jorissen, F., Fouet, M., Armynot du Chatelet, E., Barras, C., Bouchet, V., Daviray, M., Francescangeli, F., Geslin, E., Le Moigne, D., Licari, L., Mojtahid, M., Nardelli M.-P., Pavard, J.-C., Rolland, A., Schweizer, M., and Singer, D.: Foraminifères estuariens de la façade atlantique française. Guide de determination, Université d'Angers and Office Francais de la Biodiversite (in French), 89 pp., 2023.
Kankainen, M., Martinsson, S., Nordberg, K., and Polovodova Asteman, I.: In situ ecological quality status in the Kosterhavet National Park (Skagerrak, North Sea): a 100-year perspective, Ecol. Indic., 147, 110005, https://doi.org/10.1016/j.ecolind.2023.110005, 2023.
Karlsson, R., Obst, M., and Berggren, M.: Analysis of potential distribution and impacts for two species of alien crabs in Northern Europe, Biol. Invasions, 21, 3109–3119, https://doi.org/10.1007/s10530-019-02044-3, 2019.
Katsanevakis, S., Wallentinus, I., Zenetos, A., Leppäkoski, E., Çinar, M.E., Oztürk, B., Grabowski, M., Golani, D., and Cardoso, A.C.: Impacts of invasive alien marine species on ecosystem services and biodiversity: a pan-European review, Aquat. Invasions, 9, 391–423, https://doi.org/10.3391/ai.2014.9.4.01, 2014
Kitazato, H. and Matsushita, S.: Laboratory observations of sexual and asexual reproduction of Trochammina hadai Uchio, Transactions and Proceedings of the Paleontological Society of Japan. New series, 1996, 454–466, 1996.
Klein, R.: The effects of marinas och boating activity upon tidal waterways. Community and Environmental Defence Services, Maryland, 23 pp., https://tidewatercurrent.com/Marinas.pdf (last access: 19 May 2025), 2007.
Koho, K. A., Lekieffre, C., Nomaki, H., Salonen, I., Geslin, E., Mabilleau, G., Søgaard Jensen, L. H., and Reichart, G. J.: Changes in ultrastructural features of the foraminifera Ammonia spp. in response to anoxic conditions: Field and laboratory observations, Mar. Micropaleontol., 138, 72–82, https://doi.org/10.1016/j.marmicro.2017.10.011, 2018.
Korsun, S., Hald, M., Golikova, E., Yudina, A., Kuznetsov, I., Mikhailov, D., and Knyazeva, O.: Intertidal foraminiferal fauna and the distribution of Elphidiidae at Chupa Inlet, western White Sea, Mar. Biol. Res., 10, 153–166, https://doi.org/10.1080/17451000.2013.814786, 2014.
Kosakyan, A., Heger, T. J., Leander, B. S., Todorov, M., Mitchell, E. A., and Lara, E.: COI barcoding of Nebelid testate amoebae (Amoebozoa: Arcellinida): extensive cryptic diversity and redefinition of the Hyalospheniidae Schultze, Protist, 163, 415–434, https://doi.org/10.1016/j.protis.2011.10.003, 2012.
Langer, M. R., Weinmann, A. E., Loetters, S., and Roedder, D.: “Strangers” in paradise: modeling the biogeographic range expansion of the foraminifera Amphistegina in the Mediterranean Sea, J. Foramin. Res., 42, 234–244, https://doi.org/10.2113/gsjfr.42.3.234, 2012.
Lefort, V., Longueville, J. E., and Gascuel, O.: SMS: smart model selection in PhyML, Mol. Biol. Evol., 34, 2422–2424, https://doi.org/10.1093/molbev/msx149, 2017.
LeKieffre, C., Spangenberg, J. E., Mabilleau, G., Escrig, S., Meibom, A., and Geslin, E.: Surviving anoxia in marine sediments: The metabolic response of ubiquitous benthic foraminifera (Ammonia tepida), Plos One, 12, e0177604, https://doi.org/10.1371/journal.pone.0177604, 2017.
LeKieffre, C., Jauffrais, T., Geslin, E., Jesus, B., Bernhard, J. M., Giovani, M. E., and Meibom, A.: Inorganic carbon and nitrogen assimilation in cellular compartments of a benthic kleptoplastic foraminifer, Sci. Rep., 8, 10140, https://doi.org/10.1038/s41598-018-28455-1, 2018.
Linnaeus, C.: Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis, [The system of nature through the three kingdoms of nature, according to classes, orders, genera, species, with characters, differences, synonyms, places], Impensis Direct. Laurentii Salvii. Holmiae, Stockholm, 1, 824 pp., https://biodiversitylibrary.org/page/726886 (last access: 15 May 2025), 1758.
Loosanoff, V. L. and Engle, J. B.: Polydora in Oysters Suspended in the Water, Biological Bulletin, Marine Biological Laboratory, Woods Hole, 85, 69–78, http://www.biolbull.org/content/85/1/69.full.pdf+html (last access: 17 May 2025), 1943.
Maciute, A., Holovachov, O., Glud, R. N., Broman, E., Berg, P., Nascimento, F. J., and Bonaglia, S.: Reconciling the importance of meiofauna respiration for oxygen demand in muddy coastal sediments, Limnol. Oceanogr., 68, 1895–1905, https://doi.org/10.1002/lno.12393, 2023.
Matoba, Y.: Distribution of recent shallow water foraminifera of Matsushima Bay, Miyagi Prefecture, northeast Japan, The Science Reports of the Tohoku University, Second series (Geology), 42, 1–85, https://tohoku.repo.nii.ac.jp/records/12008 (last access: 17 May 2025), 1970.
Matsushita, S. and Kitazato, H.: Seasonality in the benthic foraminiferal community and the life history of Trochammina hadai Uchio in Hamana Lake, Japan, in: Paleoecology, biostratigraphy, paleoceanography and taxonomy of agglutinated foraminifera, edited by: Hemleben, C., Kaminski, M. A., Kuhnt, W., and Scott, D. B., Springer, Dordrecht, the Netherlands, 695–715, https://https://doi.org/10.1007/978-94-011-3350-0, 1990.
McGann, M. and Holzmann, M.: First Occurrence of the nonindigenous Asian foraminifera Ammonia confertitesta in the Northeastern Pacific Ocean: Vancouver Island, British Columbia, Canada, Micropaleontology, 70, 115–127, https://doi.org/10.47894/mpal.70.2.02, 2024.
McGann, M., Sloan, D., and Cohen, A. N.: Invasion by a Japanese marine microorganism in western North America, Hydrobiologia, 421, 25–30, https://doi.org/10.1023/A:1003808517945, 2000.
McGann, M., Grossman, E. E., Takesue, R. K., Penttila, D., Walsh, J. P., and Corbett, R.: Arrival and expansion of the invasive foraminifera Trochammina hadai Uchio in Padilla Bay, Washington, Northwest Sci., 86, 9–26, https://doi.org/10.3955/046.086.0102, 2012.
McGann, M., Ruiz, G. M., Hines, A. H., and Smith, G.: A Ship's Ballasting History As an Indicator of Foraminiferal Invasion Potential – an Example from Prince William Sound, Alaska, Usa, J. Foramin. Res., 49, 434–455, https://doi.org/10.2113/gsjfr.49.4.434, 2019.
McGann, M., Holzmann, M., Bouchet, V. M. P., Eichler, P. P. B., Haig, D. W., Himson, S. J., Kitazato, H., Pavard, J.-C., Polovodova Asteman, I., Rodrigues, A. R., Tremblin, C. M., Tsuchiya, M., Williams, M., O'Brien, P., Asplund, J., and Axelsson, M.: Analysis of a human-mediated microbioinvasion: the global spread of the benthic foraminifer Trochammina hadai Uchio, 1962, J. Micropalaeontol., in press, 2025.
Moksnes, P. O., Eriander, L., Hansen, J., Albertsson, J., Andersson, M., Bergström, U., Carlström, J., Egardt, J., Fredriksson, R., Granhag, L., Lindgren, F., Nordberg, K., Wendt, I., Vikström, S., and Ytreberg, E.: Fritidsbåtars påverkan på grunda kustekosystem i Sverige. Havsmiljöinstitutets Rapport nr. 2019:3, 157 s. [Impact of leisure boats on shallow-water ecosystems in Sweden. Swedish Institute for the Marine Environment, Report no. 2019:3, 157 pp.], 2019 (in Swedish with summary in English).
Montagu, G.: Testacea Britannica or natural history of British shells, marine, land, and fresh-water, including the most minute: Systematically arranged and embellished with figures, sold by White, J., London, Vol. 1, xxxvii + 291 pp., Vol. 2, 293–606, pl. 1–16, http://www.biodiversitylibrary.org/item/78694 (last access: 15 May 2025), 1803.
Morin, F., Panova, M. A. Z., Schweizer, M., Wiechmann, M., Eliassen, N., Sundberg, P., Cluzel-Burgalat, L., and Polovodova Asteman, I.: Hidden aliens: Application of digital PCR to track an exotic foraminifer across the Skagerrak (North Sea) correlates well with traditional morphospecies analysis, Environ. Microbiol., 25, 2321–2337, https://doi.org/10.1111/1462-2920.16458, 2023.
Moros, M., Andersen, T. J., Schulz-Bull, D., Häusler, K., Bunke, D., Snowball, I., Kotilainen, A., Zillén, L., Jensen, J. B., Kabel, K., Hand, I., Leipe, T., Lougheed, B. C., Wagner, B., and Arz, H. W.: Towards an event stratigraphy for Baltic Sea sediments deposited since AD 1900: approaches and challenges, Boreas, 46, 129–142, https://doi.org/10.1111/bor.12193, 2017.
Morrell, J. J. and Huffman, J.: Copper, chromium, and arsenic levels in soils surrounding posts treated with chromated copper arsenate (CCA), Wood Fiber Sci., 36, 119–128, 2004.
Murray, J. W.: The enigma of the continued use of total assemblages in ecological studies of benthic foraminifera, J. Foramin. Res., 30, 244–245, 2000.
Murray, J. W. and Alve, E.: Natural dissolution of modern shallow water benthic foraminifera: taphonomic effects on the palaeoecological record, Palaeogeogr. Palaeocl., 146, 195–209, https://doi.org/10.1016/S0031-0182(98)00132-1, 1999.
National Library of Medicine: GenBank Overview, National Library of Medicine [data set], https://www.ncbi.nlm.nih.gov/genbank/, last access: 19 May 2025.
Naturvårdsverket: Bedömningsgrunder för miljökvalitet: sjöar och vattendrag: bakgrundsrapport 1, kemiska och fysikalista parametrar. [Swedish Environmental Protection Agency: Assessment guidelines for environmental status in lakes and rivers: background report 1: chemical and physical parameters], https://viss.lansstyrelsen.se/ReferenceLibrary/50383/Bilaga_A_sjo_och_vattendrag_webb.pdf (last access: 15 May 2025), 2000 (in Swedish).
Naturvårdsverket: Alkylatbensin i småbåtsmotorer – analys av miljöfördelar, Rapport 6307 Naturvårdsverket, ISBN 978-91-620- 6307-8 [Alkylate gasoline in leisure boat motors – analysis of environmental benefits, Report 6307, ISBN 978-91-620- 6307-8], Environmental Protection Agency, https://naturvardsverket.diva-portal.org/smash/record.jsf?pid=diva2%3A1617668&dswid=8362, (last access: 15 May 2025), 2009 (in Swedish).
Nikulina, A., Polovodova, I., and Schönfeld, J.: Foraminiferal response to environmental changes in Kiel Fjord, SW Baltic Sea, eEarth, 3, 37–49, 2008.
Nordberg, K., Bornmalm, L., Cato, I., Arneborg, L., Björk, G., and Robijn, A.: Sannäsfjorden – en studie av hydrografisk, bottendynamisk och miljökemisk status [Sannäs Fjord and a study of its hydrography, bottom dynamics and environmental geochemistry], Department of Earth Science, University of Gothenburg, Report 2012 C95, 2012 (in Swedish with summary in English).
Nordberg, K., Polovodova Asteman, I., Gallagher, T. M., and Robijn, A.: Recent oxygen depletion and benthic faunal change in shallow areas of Sannäs Fjord, Swedish west coast, J. Sea Res., 127, 46–62, https://doi.org/10.1016/j.seares.2017.02.006, 2017.
Nordberg, K., Björk, G., Lundin, L., Abrahamsson, K., Josefsson, S., Dahlberg, C., Zar, I.: Fritidsbåtars avgasutsläpp i skärgårdsmiljön. Havsmiljöinstitutet, Rapport nr 2022:2. 148 pp. [Exhaust discharges of leisure boats in the coastal archipelago, Swedish Institute for the Marine Environment, Report No. 2022:2, 148 pp.], https://www.havsmiljoinstitutet.se/publikationer/havsmiljoinstitutets-rapportserie/fritidsbatars-avgasutslapp-i-skargarden (last access: 15 May 2025), 2022 (in Swedish with summary in English).
Nordberg, K., Björk, G., Abrahamsson, K., Josefsson, S., and Lundin, L.: Historic distribution of Polycyclic Aromatic Compounds (PAC) in a Skagerrak fjord, Swedish west coast as reflected in a high-resolution sediment record and compared to the Environmental Quality Standards (EQS), Mar. Pollut. Bull., 199, 116014, https://doi.org/10.1016/j.marpolbul.2023.116014, 2024.
Nordberg, K., Björk, G., Abrahamsson, K., Josefsson, S., and Lundin, L.: Tracing PAH emissions from leisure boats in a low tidal coastal area, including comparison with Environmental Quality Standards (EQS), Chemosphere, 370, 143910, https://doi.org/10.1016/j.chemosphere.2024.143910, 2025.
O'Brien, P., Barrenechea Angeles, I., Cermakova, K., Pawlowski, J., Alve, E., Nordberg, K., and Polovodova Asteman, I.: Assessing environmental quality in a historically polluted fjord: a comparison of benthic foraminiferal eDNA and morphospecies approaches, J. Geophys. Res.-Biogeo., 129, e2023JG007781, https://doi.org/10.1029/2023JG007781, 2024.
Orsi, W. D., Morard, R., Vuillemin, A., Eitel, M., Wörheide, G., Milucka, J., and Kucera, M.: Anaerobic metabolism of Foraminifera thriving below the seafloor, ISME J., 14, 2580–2594, https://doi.org/10.1038/s41396-020-0708-1, 2020.
Pavard, J. C., Bouchet, V. M., Richirt, J., Courleux, A., Armynot Du Châtelet, E., Duong, G., Abraham, R., Pezy, J.-P., Dauvin, J-C., and Seuront, L.: Preferential presence in harbours confirms the non-indigenous species status of Ammonia confertitesta (Foraminifera) in the English Channel, Aquat. Invasions, 18, 351–369, https://doi.org/10.3391/ai.2023.18.3.106635, 2023a.
Pavard, J. C., Richirt, J., Seuront, L., Blanchet, H., Fouet, M. P., Humbert, S., Gouillieux, B., Duong, G., and Bouchet, V. M.: The great shift: The non-indigenous species Ammonia confertitesta (Foraminifera, Rhizaria) outcompetes indigenous Ammonia species in the Gironde estuary (France), Estuar. Coast. Shelf S., 289, 108378, https://doi.org/10.1016/j.ecss.2023.108378, 2023b.
Pawlowski, J.: Introduction to the molecular systematics of foraminifera, Micropaleontology, 46, 1–12, https://www.jstor.org/stable/1486176 (last access: 15 May 2025), 2000.
Pawlowski, J. and Holzmann, M.: Diversity and geographic distribution of benthic foraminifera: a molecular perspective, in: Protist Diversity and Geographical Distribution, edited by: Foissner, W. and Hawksworth, D. L., Topics in Biodiversity and Conservation, vol. 8, Springer, Dordrecht, the Netherlands, 83–94, https://doi.org/10.1007/978-90-481-2801-3_7, 2008.
Pawlowski, J. and Holzmann, M.: A plea for DNA barcoding of foraminifera, J. Foramin. Res., 44, 62–67, https://doi.org/10.2113/gsjfr.44.1.62, 2014.
Petersen, J., Riedel, B., Barras, C., Pays, O., Guihéneuf, A., Mabilleau, G., Schweizer, M., Meysman, F. J. R., and Jorissen, F. J.: Improved methodology for measuring pore patterns in the benthic foraminiferal genus Ammonia, Mar. Micropaleontol., 128, 1–13, https://doi.org/10.1016/j.marmicro.2016.08.001, 2016.
Pettay, D. T., Wham, D. C., Smith, R. T., Iglesias-Prieto, R., and LaJeunesse, T. C.: Microbial invasion of the Caribbean by an Indo-Pacific coral zooxanthella, P. Natl. Acad. Sci. USA, 112, 7513–7518, https://doi.org/10.1073/pnas.1502283112, 2015.
Piña-Ochoa, E., Høgslund, S., Geslin, E., Cedhagen, T., Revsbech, N. P., Nielsen, L. P., Schweizer, M., Jorissen, F., Rysgaard, S., and Risgaard-Petersen, N.: Widespread occurrence of nitrate storage and denitrification among Foraminifera and Gromiida, P. Natl. Acad. Sci. USA, 107, 1148–1153, https://doi.org/10.1073/pnas.0908440107, 2010.
Polovodova, I., Nikulina, A., Schönfeld, J., and Dullo, W. C.: Recent benthic foraminifera in the Flensburg Fjord (western Baltic Sea), J. Micropalaeontol., 28, 131–142, https://doi.org/10.1144/jm.28.2.131, 2009.
Polovodova Asteman, I., Hanslik, D., and Nordberg, K.: An almost completed pollution-recovery cycle reflected by sediment geochemistry and benthic foraminiferal assemblages in a Swedish–Norwegian Skagerrak fjord, Mar. Pollut. Bull., 95, 126–140, https://doi.org/10.1016/j.marpolbul.2015.04.031, 2015.
Polovodova Asteman, I. and Schönfeld, J.: Recent invasion of the foraminifer Nonionella stella Cushman and Moyer, 1930 in northern European waters: evidence from the Skagerrak and its fjords, J. Micropalaeontol., 35, 20–25, 2016.
Port of Gothenburg: Official webpage https://www.portofgothenburg.com (last access: 1 June 2023), 2023.
Rao, K. K.: Ecology of Mandovi and Zuari estuaries, Goa: Distribution of foraminiferal assemblages, Indian J. Geo-Mar. Sci. (IJMS), 3, 61–66, 1974.
Rao, K. K., Jayalakshmy, K. V., Venugopal, P., Gopalakrishnan, T. C., and Rajagopal, M. D.: Foraminifera from the Chilka Lake on the east coast of India, J. Mar. Biol. Assoc. India, 42, 47–61, 2000.
Richirt, J., Schweizer, M., Bouchet, V. M., Mouret, A., Quinchard, S., and Jorissen, F. J. Morphological distinction of three Ammonia phylotypes occurring along European coasts, J. Foramin. Res., 49, 76–93, 2019.
Richirt, J., Schweizer, M., Mouret, A., Quinchard, S., Saad, S. A., Bouchet, V. M., Wade, C. M., and Jorissen, F. J.: Biogeographic distribution of three phylotypes (T1, T2 and T6) of Ammonia (foraminifera, Rhizaria) around Great Britain: new insights from combined molecular and morphological recognition, J. Micropalaeontol., 40, 61–74, https://doi.org/10.5194/jm-40-61-2021, 2021.
Rehitha, T. V., Ullas, N., Vineetha, G., Benny, P. Y., Madhu, N. V., and Revichandran, C.: Impact of maintenance dredging on macrobenthic community structure of a tropical estuary, Ocean Coast. Manage., 144, 71–82, https://doi.org/10.1016/j.ocecoaman.2017.04.020, 2017.
Rosenberg, R.: Effects of dredging operations on estuarine benthic macrofauna, Mar. Pollut. Bull., 8, 102–104, https://https://doi.org/10.1016/0025-326X(77)90131-X, 1977.
Roy, H. E., Pauchard, A., Stoett, P., Truong, T. R., Bacher, S., Galil, B. S., Hulme, P. E., Ikeda, T., Kavileveettil, S., McGeoch, M. A., Meyerson, L. A., Nuñez, M. A., Ordonez, A., Rahlao, S. J., Schwindt, E., Seebens, H., Sheppard, A. W., and Vandvik, V.: IPBES Invasive Alien Species Assessment: Summary For Policymakers, IPBES, https://zenodo.org/records/11254974 (last access: 20 February 2025), 2023.
Ruiz, G. M., Rawlings, T. K., Dobbs, F. C., Drake, L. A., Mullady, T., Huq, A., and Colwell, R. R.: Global spread of microorganisms by ships, Nature, 408, 49–50, https://doi.org/10.1038/35040695, 2000.
SAMWM: Swedish Agency for Marine and Water Management: The list of invasive and alien species in Swedish waters, https://www.havochvatten.se/arter-och-livsmiljoer/invasiva-frammande-arter/, last access: 8 January 2023 (in Swedish).
Saad, S. A. and Wade, C. M.: Biogeographic distribution and habitat association of Ammonia genetic variants around the coastline of Great Britain, Mar. Micropaleontol., 124, 54–62, https://doi.org/10.1016/j.marmicro.2016.01.004, 2016.
Salonen, I. S., Chronopoulou, P. M., Bird, C., Reichart, G. J., and Koho, K. A.: Enrichment of intracellular sulphur cycle–associated bacteria in intertidal benthic foraminifera revealed by 16S and aprA gene analysis, Sci. Rep., 9, 11692, https://doi.org/10.1038/s41598-019-48166-5, 2019.
Schurin, A.: Zur Fauna der Caprelliden der Bucht Peters der Grosse, (Japanisches Meer), Zool. Anz., 112, 198–203, 1935.
Schweizer, M., Polovodova, I., Nikulina, A., and Schönfeld, J.: Molecular identification of Ammonia and Elphidium species (Foraminifera, Rotaliida) from the Kiel Fjord (SW Baltic Sea) with rDNA sequences, Helgoland Mar. Res., 65, 1–10, https://doi.org/10.1007/s10152-010-0194-3, 2011.
Schweizer, M., Jauffrais, T., Choquel, C., Méléder, V., Quinchard, S., and Geslin, E.: Trophic strategies of intertidal foraminifera explored with single-cell microbiome metabarcoding and morphological methods: What is on the menu?, Ecol. Evol., 12, e9437, https://doi.org/10.1002/ece3.9437, 2022.
Shukla, K., Kumar, B., Agrawal, R., Priyanka, K., Venkatesh, M., and Anshumali: Assessment of Cr, Ni and Pb pollution in rural agricultural soils of Tonalite–Trondjhemite Series in Central India, B. Environ. Contam. Tox., 98, 856–866, https://doi.org/10.1007/s00128-017-2085-7, 2017.
Skjevik, A.-T.: Växtplanktonrapport 2004/ Phytoplankton report, The Swedish Meteorological and Hydrological Institute (SMHI) Report 2005 – 09, 12 pp., https://www.bvvf.se/download/18.6ab8a0381529bd4d34d6365d/1454398232645/2004.%20V%E4xtplankton_2004_SMHI.pdf, (last access: 17 May 2025), 2004 (in Swedish).
Simkanin, C., Davidson, I., Falkner, M., Sytsma, M., and Ruiz, G.: Intra-coastal ballast water flux and the potential for secondary spread of non-native species on the US West Coast, Mar. Pollut. Bull., 58, 366–374, https://doi.org/10.1016/j.marpolbul.2008.10.013, 2009.
Staehr, P. A., Jakobsen, H. H., Hansen, J. L., Andersen, P., Christensen, J., Göke, C., Thomsen, M. S., and Stebbing, P. D.: Trends in records and contribution of non-indigenous and cryptogenic species to marine communities in Danish waters: potential indicators for assessing impact, Aquat. Invasions, 15, 217–244, https://doi.org/10.3391/ai.2020.15.2.02, 2020.
Sveriges Geologiska Undersökning (SGU): Kartblad, SGU Serie Ba nr 59:4, Maringeologiska kartan: Ytsedimentfördelning i Göteborgs stad och Öckerö kommun, skala 1:50000 [SGU Map Series Ba nr 59:4, Marine geological map: seabed sediments in Gothenburg City and Öckerö Municipality, scale 1:50000], https://resource.sgu.se/dokument/publikation/ba/ba59karta/ba59-karta.pdf (last access: 20 May 2025), 2002.
Sveriges Geologiska Undersökning (SGU)/Swedish Geological Survey: Map viewer: Marine geology, https://apps.sgu.se/kartvisare/kartvisare-maringeologi.html, last access: 5 June 2023.
Swedish Environmental Institute (Svenska miljöinstitutet): Nytt invasivt skadedjur upptäckt i svenska ostronbankar, Pressmeddelande 2020-11-05 [The Swedish Environmental Institute: New invasive vermin has been discovered in Swedish oyster banks, Press release 2020-11-05], https://www.ivl.se/toppmeny/press/pressmeddelanden-och-nyheter/pressmeddelanden/2020-11-05-nytt-invasivt-skadedjur-upptackt-i-svenska-ostronbankar.html#container2020 (last access: 8 January 2024), 2020 (in Swedish).
Tango, P., Butler, W., Wazniak, C., and Hall, M.: Assessment of harmful algae bloom species in the Maryland Coastal Bays, in: Maryland’s Coastal Bays Ecosystem Health Assessment 2004, edited by: Wazniak, C., Goshorn, D., Hall, M., Blazer, D., Jesien, R., Wilson, D., Cain, C., Dennison, W., Thomas, J., Carruthers, T., And Sturgis, B., Maryland Department of Natural Resources, 8-2–8-34, https://dnr.maryland.gov/waters/coastalbays/Documents/EHA_2004.pdf (last access: 17 May 2025), 2004
Thomas, K. V. and Brooks, S.: The environmental fate and effects of antifouling paint biocides, Biofouling, 26, 73–88, https://doi.org/10.1080/08927010903216564, 2010.
Thunberg, C. P.: Tekning och Beskrifning på en stor Ostronsort ifrån Japan, Kongliga Vetenskaps Academiens Nya Handlingar, 14, 140–142, https://www.biodiversitylibrary.org/page/46957303 (last access: 17 May 2025), 1793.
Toriumi, S. and Takano, H.: A new genus of the Chloromonadophyceae from Atsumi Bay, Japan. Bull. Tokai Reg. Fish. Res. Lab., 76, 25–35, 1973.
Transportstyrelsen: Båtlivsundersökningen 2015: en undersökning om svenska fritidsbåtar och hur de används. Rapport no. TSG 2016e2534, mars 2016, 122 s. [The Swedish Transport Agency (2016). Leisure boat survey 2015: an investigation about Swedish leisure boats and how they are used. Report nr. TSG 2016e2534, March 2016, 122 pp.], https://www.transportstyrelsen.se/globalassets/global/sjofart/dokument/fritidsbatar1/transportstyrelsen-batlivsundersokning-2015.pdf, (last access: 17 May 2025), 2016 (in Swedish).
Transportstyrelsen: Båtlivsundersökningen 2020: en undersökning om svenska fritidsbåtar och hur de används. Rapport Båtlivsundersökningen 2020, Transportstyrelsen, Dnr 2021-2170, 108 s. [The Swedish Transport Agency (2021). Leisure boat survey 2020: an investigation about Swedish leisure boats and how they are used. Report nr. 2021-2170, 108 pp.], https://www.transportstyrelsen.se/globalassets/global/sjofart/dokument/fritidsbatar1/transportstyrelsen-batlivsundersokningen-2020.pdf (last access: 17 May 2025), 2021 (in Swedish).
Tremblin, C. M., Holzmann, M., Parker, J. H., Sadekov, A., and Haig, D. W.: Invasive Japanese foraminifera in a south-west Australian estuary, Mar. Freshwater Res., 73, 328–342, https://doi.org/10.1071/MF21254, 2021.
Turner, A.: Marine pollution from antifouling paint particles, Mar. Pollut. Bull., 60, 159–171, https://doi.org/10.1016/j.marpolbul.2009.12.004, 2010.
Uchio, T.: Influence of the River Shinano on foraminifera and sediment grain size distributions, Seto Marine Laboratory, Kyoto University, Sirahama, Japan, 10, 363–392, https://repository.kulib.kyoto-u.ac.jp/dspace/bitstream/2433/175306/1/fia0102_363.pdf (last access: 20 May 2025), 1962.
Weiss, L.: Foraminifera and origin of the Gardiners clay (Pleistocene), eastern Long Island, New York, United States Geological Survey Professional Papers, 254-G, 143–163, https://doi.org/10.3133/pp254g, 1954.
Wetzel, R. G.: Sediments and microflora, in: Limnology Lake and River Ecosystems, edited by: Wetzel, R .G., 631–664, https://doi.org/10.1016/B978-0-08-057439-4.50025-3, 2001.
Zheng, S. and Fu, Z. X.: Faunal trends and assemblages of the northern South China Sea agglutinated foraminifera, in: Paleoecology, Biostratigraphy, Paleoceanography and Taxonomy of Agglutinated Foraminifera, edited by: Hemleben, C., Kaminski, M. A., Kuhnt, W., and Scott, D. B., Springer, Dordrecht, the Netherlands, 541–563, https://doi.org/10.1007/978-94-011-3350-0_19, 1990.
Zheng, S. Y., Cheng, T., Wang, X., and Fu, Z.: The Quaternary Foraminifera of the Dayuzhang Irrigation area, Shandong Province, and a preliminary attempt at an interpretation of its depositional environment, Studia Marina Sinica, 13, 72–78, 1978.